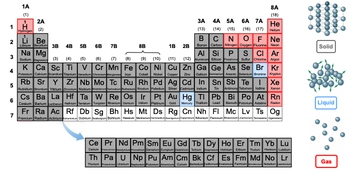
As we delve into the periodic table, it's essential to understand how elements exist in different states of matter at standard room temperature (approximately 25 degrees Celsius) and standard pressure (1 atmosphere). The three primary states are solids, liquids, and gases, each characterized by distinct molecular arrangements and behaviors.
In solids, molecules are tightly packed and locked in place, resulting in a fixed shape and volume. This rigidity means that solids do not conform to the shape of their container. For example, a block of ice retains its shape regardless of the container it is placed in.
Liquids, on the other hand, have molecules that are close together but can move around more freely. This allows liquids to take the shape of their container while maintaining a constant volume. For instance, if a liquid occupies only 10 mL in a 25 mL container, it will fill the bottom of the container but not the entire volume.
Gases exhibit a different behavior; their molecules are spaced far apart, which allows them to expand and fill both the shape and volume of their container. This property is a significant distinction from solids and liquids, as gases can compress and occupy varying amounts of space depending on the container.
When examining the periodic table, most elements are found in solid form, such as lithium, zinc, and sulfur. A few elements exist as gases, including hydrogen, nitrogen, oxygen, and chlorine, along with the noble gases in group 8A, which are inherently gaseous. The rarest state is liquid, with only two elements—mercury and bromine—existing as liquids under standard conditions.
Additionally, some elements, particularly those in the seventh row (from francium to oganesson), are synthetically produced in laboratories. Due to their high atomic masses and instability, their states at standard temperature and pressure are unpredictable, which is why they are not assigned a specific state of matter.
In summary, the state of matter for each element is influenced by temperature and pressure, leading to a diverse array of physical properties across the periodic table.