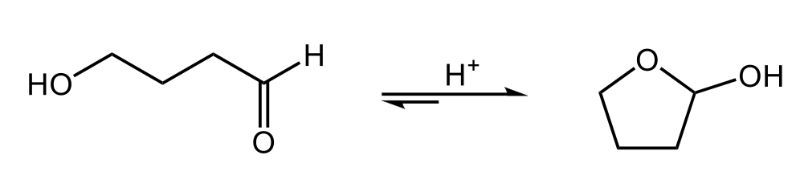
Cyclic hemiacetals are formed when an aldehyde or ketone reacts with an alcohol, resulting in a structure that features a carbon atom previously part of a carbonyl group now bonded to both a hydroxyl group (–OH) and an alkoxy group (–OR). This reaction involves one mole of alcohol interacting with the carbonyl compound, leading to the creation of a hemiacetal.
In the case of acyclic hemiacetals, which are not part of a ring structure, they are inherently unstable. The reaction tends to favor the original reactants—aldehyde and alcohol—over the formation of the hemiacetal. This is illustrated by the reaction arrows, where smaller arrows indicate the forward reaction to form the hemiacetal, while a larger arrow points back to the reactants, signifying a strong preference for the reactant state.
To clarify, a hemiacetal is characterized by a carbon atom that was once part of a carbonyl group (double-bonded to oxygen) and is now single-bonded to an –OH group and an –OR group, where R represents a carbon-containing group. The instability of acyclic hemiacetals means they readily revert to their original aldehyde and alcohol forms, making them less favorable in equilibrium.
Understanding the distinction between acyclic and cyclic hemiacetals is crucial, as the latter can exhibit greater stability due to their ring structure, which will be explored further in subsequent discussions.