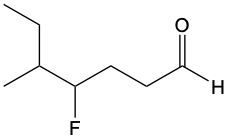
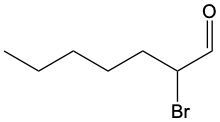
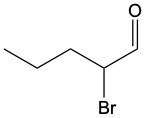
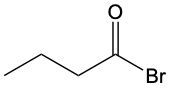
Aldehydes are organic compounds characterized by the presence of a carbonyl group (C=O) where the carbonyl carbon is directly bonded to a hydrogen atom. When naming aldehydes, the rules are similar to those for ketones, with a key distinction: the carbonyl carbon in aldehydes is always assigned the number 1. This means that when determining the longest carbon chain, the numbering begins at the carbonyl carbon.
In the naming convention for aldehydes, the suffix of the parent alkane name is modified from "-e" to "-al" to indicate the presence of the aldehyde functional group. For example, the alkane "pentane" becomes "pentanal" when it is converted to an aldehyde. Additionally, it is essential to provide the numerical locations of any substituents on the carbon chain, ensuring that the parent name reflects these modifications accurately.
As you explore various aldehydes, remember to apply these naming rules consistently, starting with the carbonyl carbon and adjusting the suffix accordingly. This systematic approach will help in identifying and naming aldehydes correctly in organic chemistry.