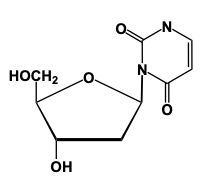
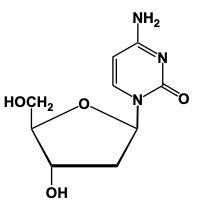
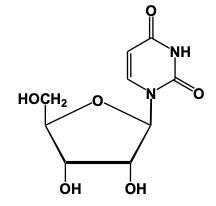
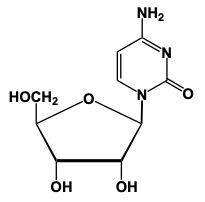
Nucleoside formation involves the creation of a glycosidic bond between a sugar and a nitrogenous base. This process begins with the anomeric carbon, which is carbon number 1 of the sugar, interacting with the nitrogen of the nitrogenous base. For instance, when ribose sugar, characterized by an -OH group on carbon number 2, reacts with uracil, a nitrogenous base, a condensation reaction occurs. In this reaction, a water molecule is lost as the -OH group from the sugar and an -H from the nitrogenous base combine to form water.
As a result of this reaction, the carbon and nitrogen atoms form a new bond, creating the glycosidic bond that links the sugar and the base. The outcome is a nucleoside, which consists of a pentose sugar and a nitrogenous base. In the case of ribose and uracil, the product is a nucleoside that retains the pentose ring structure.
When considering 2-deoxyribose, which lacks the -OH group on carbon number 2, the process is similar. Here, the sugar reacts with adenine, a purine nitrogenous base. Again, a condensation reaction occurs, resulting in the loss of water and the formation of a glycosidic bond between the sugar and the base. This process highlights the importance of the anomeric carbon in nucleoside formation, as it is crucial for the interaction with the nitrogen of the base, leading to the synthesis of nucleosides essential for nucleic acid structure.