In organic chemistry, the systematic naming of compounds follows the IUPAC (International Union of Pure and Applied Chemistry) nomenclature rules. When it comes to naming alkanes, which are saturated hydrocarbons, the names are derived from the number of carbon atoms in the molecule, with all alkane names ending in the suffix -ane.
The alkane prefixes correspond to the number of carbon atoms present, ranging from one to ten for introductory chemistry. Here’s a breakdown of the prefixes and their corresponding alkane names:
- 1 carbon: Meth - Methane
- 2 carbons: Eth - Ethane
- 3 carbons: Prop - Propane
- 4 carbons: But - Butane
- 5 carbons: Pent - Pentane
- 6 carbons: Hex - Hexane
- 7 carbons: Hept - Heptane
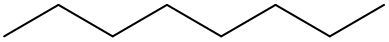
- 8 carbons: Oct - Octane
- 9 carbons: Non - Nonane
- 10 carbons: Dec - Decane
These prefixes are essential for constructing the names of alkanes, as they provide a clear indication of the number of carbon atoms in the molecule. Understanding these terms is crucial for further studies in organic chemistry, especially when dealing with larger carbon chains beyond ten carbons.