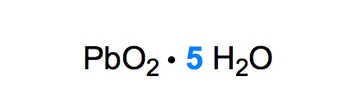Ionic hydrates are a specific type of ionic compound that are associated with one or more molecules of water. For example, in the formula \( \text{Cu}_x\text{H}_2\text{O} \), the variable \( x \) indicates the number of water molecules bonded to the copper compound. The dot in the formula signifies the connection between the ionic compound and the water molecules.
To name ionic hydrates, we follow a set of rules that are similar to those used for naming standard ionic compounds. The first three steps in the naming process mirror the established rules for ionic compounds. If you need a refresher on these rules, reviewing the relevant materials on naming ionic compounds is recommended.
The fourth step introduces a new concept: the term "hydrate" is used to refer to the water component of the ionic hydrate. Additionally, it is essential to specify the number of water molecules present. This is done using numerical prefixes that indicate the quantity of water molecules. The prefixes are as follows:
- Mono- for 1
- Di- for 2
- Tri- for 3
- Tetra- for 4
- Penta- for 5
- Hexa- for 6
- Hepta- for 7
- Octa- for 8
- Nona- for 9
- Deca- for 10
With this understanding of ionic hydrates and their naming conventions, you can now apply these rules to practice problems and enhance your comprehension of the topic.