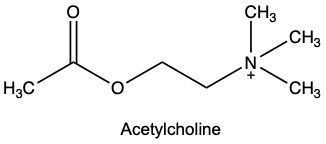
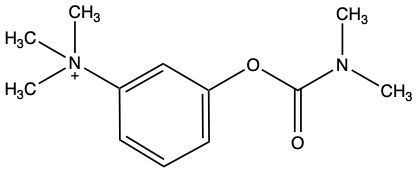
Enzyme inhibition is a crucial concept in biochemistry, referring to the process where the activity of an enzyme is decreased, leading to a reduction in the rate of catalyzed reactions. Inhibitors, which are typically small molecules or ions, bind to enzymes and interfere with their function. Understanding the types of inhibition is essential for grasping how enzymes can be regulated.
Inhibition can be categorized into two main types: competitive and noncompetitive. In competitive inhibition, the inhibitor competes with the substrate for binding to the enzyme's active site. This blockage prevents the substrate from accessing the active site, thereby reducing the reaction rate. Conversely, in noncompetitive inhibition, the inhibitor binds to a site other than the active site, known as the allosteric site. This binding alters the enzyme's shape or function, inhibiting its activity regardless of substrate presence.
Additionally, inhibition can be classified as reversible or irreversible. Reversible inhibition allows the enzyme to regain its activity once the inhibitor is removed, meaning the inhibition is temporary. In contrast, irreversible inhibition results in a permanent loss of enzyme activity, as the inhibitor forms a stable bond with the enzyme, effectively disabling it.
Overall, understanding these mechanisms of enzyme inhibition is vital for applications in drug design and metabolic regulation, as they highlight how enzyme activity can be modulated in various biological processes.