Alkenes and alkynes are known to undergo addition reactions, which involve the addition of atoms to pi bonds, leading to the breaking of double or triple bonds. In these reactions, pi bonds are broken, and new sigma bonds are formed. There are three primary types of addition reactions: halogenation, hydrogenation, and hydrohalogenation.
Halogenation involves the addition of halogens to the pi bonds of alkenes. For instance, when an alkene is treated with halogens, one of the pi bonds is broken, allowing the halogens to attach to the carbon atoms, resulting in a compound known as a dihalide. This process emphasizes the importance of carbon's tetravalency, as each carbon atom must form four bonds.
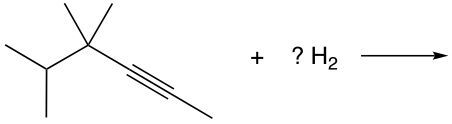
Hydrogenation, on the other hand, refers to the addition of hydrogen atoms to the pi bond of an alkene. This reaction transforms the alkene into an alkane, as two hydrogen atoms are added, while the existing hydrogen atoms remain attached to the carbon skeleton. The overall result is a saturated hydrocarbon.
Hydrohalogenation is slightly different, as it involves the addition of a hydrogen atom and a halogen atom (HX) to the alkene. In this case, the alkene reacts with a hydrogen halide, such as hydrogen bromide or hydrogen chloride, resulting in the formation of an alkyl halide. The specific distribution of hydrogen and halogen across the double bond will be determined by future rules that govern regioselectivity.
It is essential to note that one mole of reagent is required for each pi bond present. For example, an alkene, which contains one pi bond, requires one mole of reagent, while a triple bond, consisting of two pi bonds, would necessitate two moles of reagent. Understanding the structure of these bonds is crucial: a double bond comprises one sigma bond and one pi bond, while a triple bond consists of one sigma bond and two pi bonds. The sigma bond remains constant, with the number of pi bonds varying based on the type of bond present.
In summary, the three types of addition reactions—halogenation, hydrogenation, and hydrohalogenation—play a significant role in the chemistry of alkenes and alkynes, facilitating the transformation of unsaturated hydrocarbons into more saturated forms.