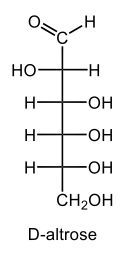
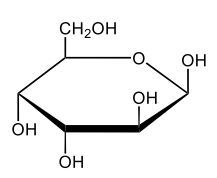
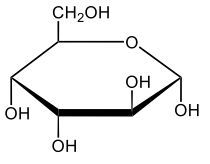
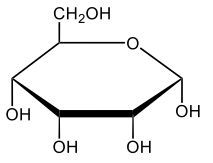
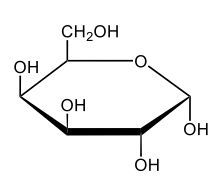
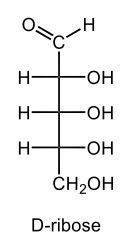
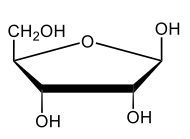
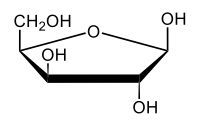
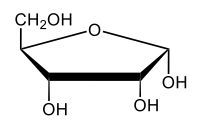
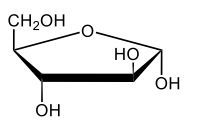
Monosaccharides, such as D-glucose, predominantly exist as cyclic hemiacetals in aqueous solutions. This cyclization occurs when the penultimate alcohol group reacts with the aldehyde group, leading to the formation of a cyclic structure. In the case of D-glucose, the cyclization results in two distinct forms based on the orientation of the hydroxyl group (–OH) at the anomeric carbon, which is the first carbon in the ring structure.
These two forms are known as anomers, which are a specific type of epimer. An epimer is defined as a pair of sugars that differ in configuration at only one chiral center. In the case of D-glucose, the anomeric carbon can have the hydroxyl group oriented either down (α-anomer) or up (β-anomer). When the –OH group is positioned opposite to the CH2OH group at carbon 6, it is referred to as the α-D-glucose. Conversely, when both groups are on the same side, it is termed β-D-glucose.
To summarize, the cyclization of D-glucose in an aqueous environment leads to the formation of two cyclic hemiacetal forms: α-D-glucose and β-D-glucose. The distinction between these anomers is crucial in carbohydrate chemistry, as it influences the properties and reactivity of the sugars involved.