Reduction of aldehydes and ketones involves the addition of hydrogen atoms, effectively increasing the number of carbon-hydrogen (C-H) bonds while maintaining the integrity of carbon-carbon (C-C) bonds. The process begins with the most oxidized form of carbon, carbon dioxide (CO2), where carbon is bonded to four oxygen atoms. As reduction occurs, one of these carbon-oxygen bonds is broken to allow the addition of hydrogen, which is essential for carbon to maintain its tetravalency (four bonds).
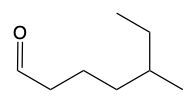
In the reduction process, as we transition from carbon dioxide to aldehydes, we systematically add hydrogen atoms. For instance, when reducing carbon dioxide to an aldehyde, we break a carbon-oxygen bond and introduce a hydrogen atom to the carbon, while also adding a hydrogen to the oxygen to satisfy its bonding requirements. This results in the formation of an aldehyde.
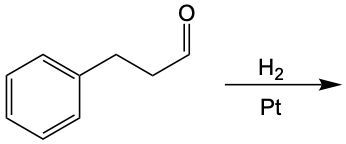
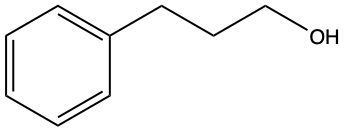
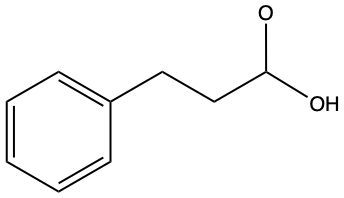
Further reduction of the aldehyde can yield an alcohol. This involves breaking another carbon-oxygen bond and adding hydrogen atoms to both the carbon and the oxygen. The general reaction can be summarized as follows:
For aldehydes:
RCHO + H2 → RCH2OH (alcohol)
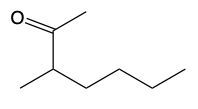
Similarly, ketones can also undergo reduction to form alcohols. The process is analogous; a ketone, which has two carbon groups attached to the carbonyl carbon, can be reduced by breaking a carbon-oxygen bond and adding hydrogen atoms:
For ketones:
RC(=O)R' + H2 → RCH(OH)R' (alcohol)
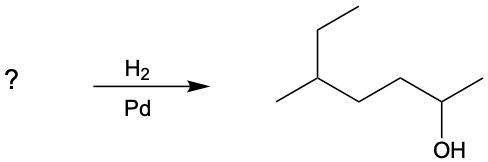
Ultimately, both aldehydes and ketones can be reduced to their corresponding alcohols, demonstrating the versatility of reduction reactions in organic chemistry. If the reduction continues, it is possible to convert alcohols into alkanes, representing the final stage of the reduction process.