Sphingolipids are a class of lipids characterized by their sphingosine backbone, which is an 18-carbon amino alcohol. The structure of sphingosine is notable for its unique carbon numbering, where the first three carbons (1 to 3) are analogous to glycerol, but the numbering is inverted. In this structure, carbon 1 is at the bottom, moving upwards to carbon 3. Specifically, carbons 1 and 3 possess hydroxyl (–OH) groups, while the amino group (–NH2) is located at carbon 2. Additionally, a long hydrocarbon chain of 15 carbons is attached to carbon 3, completing the 18-carbon structure of sphingosine. A key feature of sphingosine is the presence of a trans double bond at carbon 4, which contributes to its unique properties. Understanding these characteristics is essential for exploring the broader category of sphingolipids and their biological significance.
- 1. Matter and Measurements4h 29m
- What is Chemistry?7m
- The Scientific Method9m
- Classification of Matter16m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature (Simplified)9m
- Scientific Notation13m
- SI Units (Simplified)5m
- Metric Prefixes24m
- Significant Figures (Simplified)11m
- Significant Figures: Precision in Measurements7m
- Significant Figures: In Calculations19m
- Conversion Factors (Simplified)15m
- Dimensional Analysis22m
- Density12m
- Specific Gravity9m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects8m
- 2. Atoms and the Periodic Table5h 22m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)18m
- Atomic Mass (Conceptual)12m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Elemental Forms (Simplified)6m
- Periodic Table: Phases (Simplified)8m
- Law of Definite Proportions9m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)22m
- Electron Arrangements5m
- The Electron Configuration: Condensed4m
- The Electron Configuration: Exceptions (Simplified)12m
- Ions and the Octet Rule9m
- Ions and the Octet Rule (Simplified)8m
- Valence Electrons of Elements (Simplified)5m
- Lewis Dot Symbols (Simplified)7m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- 3. Ionic Compounds2h 18m
- Periodic Table: Main Group Element Charges12m
- Periodic Table: Transition Metal Charges5m
- Periodic Trend: Ionic Radius (Simplified)5m
- Periodic Trend: Ranking Ionic Radii8m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)8m
- Ionic Bonding6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- 4. Molecular Compounds2h 18m
- Covalent Bonds6m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Bonding Preferences6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Multiple Bonds4m
- Multiple Bonds (Simplified)6m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)8m
- Molecular Geometry (Simplified)11m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)15m
- Molecular Polarity (Simplified)7m
- 5. Classification & Balancing of Chemical Reactions3h 17m
- Chemical Reaction: Chemical Change5m
- Law of Conservation of Mass5m
- Balancing Chemical Equations (Simplified)13m
- Solubility Rules16m
- Molecular Equations18m
- Types of Chemical Reactions12m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Spontaneous Redox Reactions8m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Balancing Redox Reactions (Simplified)13m
- Galvanic Cell (Simplified)16m
- 6. Chemical Reactions & Quantities2h 34m
- 7. Energy, Rate and Equilibrium3h 40m
- Nature of Energy6m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Bond Energy14m
- Thermochemical Equations12m
- Heat Capacity19m
- Thermal Equilibrium (Simplified)8m
- Hess's Law23m
- Rate of Reaction11m
- Energy Diagrams12m
- Chemical Equilibrium7m
- The Equilibrium Constant14m
- Le Chatelier's Principle20m
- Solubility Product Constant (Ksp)17m
- Spontaneous vs Nonspontaneous Reactions7m
- Entropy (Simplified)9m
- Gibbs Free Energy (Simplified)18m
- 8. Gases, Liquids and Solids3h 27m
- Pressure Units6m
- Kinetic Molecular Theory14m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws17m
- Chemistry Gas Laws: Combined Gas Law12m
- Standard Temperature and Pressure14m
- Dalton's Law: Partial Pressure (Simplified)13m
- Gas Stoichiometry18m
- Intermolecular Forces (Simplified)19m
- Intermolecular Forces and Physical Properties11m
- Atomic, Ionic and Molecular Solids10m
- Heating and Cooling Curves30m
- 9. Solutions4h 27m
- Solutions6m
- Solubility and Intermolecular Forces17m
- Solutions: Mass Percent6m
- Percent Concentrations10m
- Molarity18m
- Osmolarity15m
- Parts per Million (ppm)13m
- Solubility: Temperature Effect8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- Dilutions12m
- Solution Stoichiometry14m
- Electrolytes (Simplified)13m
- Equivalents11m
- Molality15m
- The Colligative Properties15m
- Boiling Point Elevation16m
- Freezing Point Depression9m
- Osmosis16m
- Osmotic Pressure10m
- Vapor Pressure Lowering (Raoult's Law)16m
- 10. Acids and Bases3h 10m
- Acid-Base Introduction11m
- Arrhenius Acid and Base6m
- Bronsted Lowry Acid and Base21m
- Acid and Base Strength17m
- Ka and Kb16m
- The pH Scale16m
- Auto-Ionization9m
- pH of Strong Acids and Bases9m
- Acid-Base Equivalents14m
- Acid-Base Reactions7m
- Gas Evolution Equations (Simplified)6m
- Ionic Salts (Simplified)11m
- Buffers11m
- Henderson-Hasselbalch Equation16m
- Strong Acid Strong Base Titrations (Simplified)13m
- 11. Nuclear Chemistry1h 1m
- BONUS: Lab Techniques and Procedures1h 38m
- BONUS: Mathematical Operations and Functions47m
- 12. Introduction to Organic Chemistry1h 34m
- 13. Alkenes, Alkynes, and Aromatic Compounds2h 12m
- 14. Compounds with Oxygen or Sulfur1h 6m
- 15. Aldehydes and Ketones1h 1m
- 16. Carboxylic Acids and Their Derivatives1h 11m
- 17. Amines40m
- 18. Amino Acids and Proteins1h 51m
- 19. Enzymes1h 37m
- 20. Carbohydrates1h 46m
- Intro to Carbohydrates4m
- Classification of Carbohydrates4m
- Fischer Projections4m
- Enantiomers vs Diastereomers7m
- D vs L Enantiomers9m
- Cyclic Hemiacetals8m
- Intro to Haworth Projections4m
- Cyclic Structures of Monosaccharides11m
- Mutarotation4m
- Reduction of Monosaccharides10m
- Oxidation of Monosaccharides7m
- Glycosidic Linkage14m
- Disaccharides7m
- Polysaccharides8m
- 21. The Generation of Biochemical Energy2h 9m
- 22. Carbohydrate Metabolism2h 23m
- 23. Lipids2h 26m
- Intro to Lipids6m
- Fatty Acids25m
- Physical Properties of Fatty Acids6m
- Waxes4m
- Triacylglycerols12m
- Triacylglycerol Reactions: Hydrogenation8m
- Triacylglycerol Reactions: Hydrolysis13m
- Triacylglycerol Reactions: Oxidation7m
- Glycerophospholipids15m
- Sphingomyelins13m
- Steroids15m
- Cell Membranes7m
- Membrane Transport10m
- 24. Lipid Metabolism1h 45m
- 25. Protein and Amino Acid Metabolism1h 37m
- 26. Nucleic Acids and Protein Synthesis2h 54m
- Intro to Nucleic Acids4m
- Nitrogenous Bases16m
- Nucleoside and Nucleotide Formation9m
- Naming Nucleosides and Nucleotides13m
- Phosphodiester Bond Formation7m
- Primary Structure of Nucleic Acids11m
- Base Pairing10m
- DNA Double Helix6m
- Intro to DNA Replication20m
- Steps of DNA Replication11m
- Types of RNA10m
- Overview of Protein Synthesis4m
- Transcription: mRNA Synthesis9m
- Processing of pre-mRNA5m
- The Genetic Code6m
- Introduction to Translation7m
- Translation: Protein Synthesis18m
Sphingomyelins: Videos & Practice Problems
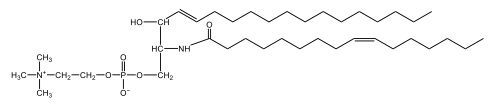
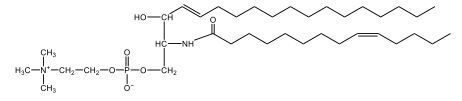
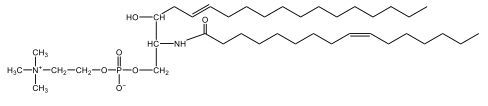
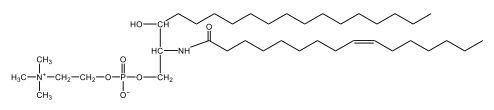
Sphingolipids are unique lipids characterized by a sphingosine backbone, an 18-carbon amino alcohol. Sphingomyelins, a type of phospholipid, consist of a sphingosine backbone, one fatty acid, a phosphate group, and a choline head. The amide bond links the fatty acid to the sphingosine. Sphingomyelins are crucial for forming the myelin sheath, which insulates nerve fibers, enhancing signal transmission. Understanding these structures is essential for grasping cellular membrane dynamics and neurological function.
Sphingomyelins Concept 1
Sphingomyelins Concept 1 Video Summary

Sphingomyelins Concept 2
Sphingomyelins Concept 2 Video Summary
Sphingomyelins are a type of phospholipid characterized by a sphingosine backbone, which is essential for their structure and function. Each sphingomyelin molecule consists of one fatty acid and features a phosphate group along with a choline head group. The structure can be visualized as having two tails: one is the sphingosine chain, while the other is a fatty acid linked through an amide bond.
In detail, the sphingosine backbone is crucial, with the phosphate group attached to carbon number 1. This phosphate is connected to an ethyl group, and the choline head group includes a nitrogen atom bonded to three methyl groups (CH3), resulting in a positively charged structure due to nitrogen's tetravalency. On carbon number 2, the amide bond is formed, where a carbonyl group is directly linked to a nitrogen atom, highlighting the importance of this bond in the overall structure.
Importantly, sphingomyelins serve as primary structural components of the myelin sheath, which insulates nerve fibers. This insulation is vital for the efficient transmission of electrical signals in the nervous system. Understanding the structure and function of sphingomyelins is essential, especially when discussing their role in nerve fiber coating and muscle function.
Sphingomyelins Example 1
Sphingomyelins Example 1 Video Summary
Sphingomyelins are a class of sphingolipids that play a crucial role in the structural integrity of the myelin sheath, which insulates nerve fibers. They are characterized by their unique backbone, which is sphingosine, rather than glycerol as seen in other lipid types. In sphingomyelins, a fatty acid is attached to the sphingosine backbone at carbon 2 through an amide linkage, contributing to their structural properties.
It is important to note that sphingomyelins possess more than one tail. While they do have a fatty acid chain at carbon 2, they also feature a hydrocarbon tail at carbon 3, which may include a trans double bond. This distinction is essential in understanding their structure and function.
When evaluating statements about sphingomyelins, one must recognize that the assertion claiming sphingomyelins have only one tail due to the presence of a single fatty acid is incorrect. This highlights the complexity of their structure, which includes multiple tails and functional groups that contribute to their biological roles.
Sphingomyelins Example 2
Sphingomyelins Example 2 Video Summary
Sphingomyelins are a class of sphingolipids that play crucial roles in cell membrane structure and function. To draw a sphingomyelin containing oleic acid, we start with the sphingosine backbone, which consists of a long-chain amino alcohol. The structure of sphingomyelin includes a phosphate group and a fatty acid chain.
First, we identify oleic acid, an unsaturated fatty acid represented by the shorthand notation 18:1. This indicates that oleic acid has an 18-carbon chain with one double bond, typically located at the ninth carbon. The drawing process begins with the sphingosine backbone:
- Draw the sphingosine backbone, placing a phosphate group at carbon number 1. This phosphate group is essential for the formation of the sphingomyelin structure.
- At carbon number 2, instead of a standard amine group (NH2), we place an NH group, which allows for the attachment of the fatty acid.
- Next, we extend the phosphate group at carbon number 1 with a choline head group. Choline consists of an ethyl group attached to an oxygen, with a nitrogen atom bonded to three methyl groups (CH3). This nitrogen atom carries a positive charge due to its four bonds.
- Finally, we draw the fatty acyl group, which is the oleic acid without the hydroxyl (OH) group, attached to the NH group at carbon number 2. This forms an amide bond. The structure of oleic acid includes 18 carbon atoms, with the double bond located at carbon 9, resulting in a trans configuration.
In summary, the final structure of sphingomyelin includes the sphingosine backbone with a phosphate group at carbon 1, an NH group at carbon 2 forming an amide bond with oleic acid, and a choline head group. The overall structure is vital for the integrity and functionality of cell membranes, highlighting the importance of sphingomyelins in biological systems.
Draw a sphingomyelin that contains palmitoleic acid.
Which one of the following statements describes how sphingomyelins are similar to glycerophospholipids?
Sphingomyelins have the same number of fatty acids as glycerophospholipids.
Sphingomyelins and glycerophospholipids have the same linkage that holds the fatty acids.
Sphingomyelins and glycerophospholipids have phosphate with head groups.
Both can be classified as sphingolipids.
Which one of the following statements is incorrect about triacylglycerols and phospholipids?
Triacylglycerols contain glycerol while phospholipids do not.
Phospholipids have a phosphate group attached at C3.
Phospholipids and triacylglycerols contain 2 and 3 fatty acids, respectively.
Due to polar head groups, phospholipids have a higher water solubility than triacylglycerols.
Do you want more practice?
Here’s what students ask on this topic:
Sphingomyelins are phospholipids characterized by a sphingosine backbone, which is an 18-carbon amino alcohol. The structure includes one fatty acid attached via an amide bond, a phosphate group, and a choline head group. The sphingosine chain forms one tail, while the fatty acid forms the other. The phosphate group is connected to the choline head, which consists of a nitrogen atom bonded to three methyl groups (CH3). This unique structure is crucial for the formation of the myelin sheath, which insulates nerve fibers and enhances signal transmission.
Sphingomyelins are essential components of the myelin sheath, the protective covering that insulates nerve fibers. This insulation is crucial for the efficient transmission of electrical signals along the nerves. The myelin sheath allows for faster signal propagation and prevents signal loss, ensuring proper neurological function. Without sufficient sphingomyelins, the integrity of the myelin sheath would be compromised, leading to potential neurological disorders.
Sphingomyelins differ from other phospholipids primarily in their backbone structure. While most phospholipids have a glycerol backbone, sphingomyelins have a sphingosine backbone, an 18-carbon amino alcohol. Additionally, sphingomyelins have one fatty acid attached via an amide bond, whereas other phospholipids typically have two fatty acids attached via ester bonds. The presence of a choline head group and a phosphate group further distinguishes sphingomyelins from other phospholipids.
The amide bond in sphingomyelins is significant because it links the fatty acid to the sphingosine backbone. This bond is formed between the carbonyl group of the fatty acid and the amino group of the sphingosine. The amide bond provides structural stability to the sphingomyelin molecule, which is crucial for its role in forming the myelin sheath. This stability ensures that the myelin sheath can effectively insulate nerve fibers and facilitate efficient signal transmission.
Sphingosine is an 18-carbon amino alcohol that serves as the backbone for sphingolipids. Key characteristics include: carbons 1 to 3 are analogous to glycerol, with hydroxyl groups (OH) on these carbons; an amino group (NH2) at carbon 2; a long chain of 15 carbons attached to carbon 3; and a trans double bond at carbon 4. These features make sphingosine unique and essential for the structure and function of sphingolipids, including sphingomyelins.