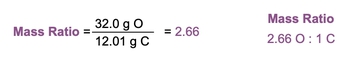The law of definite proportions, also known as Proust's law or the law of constant composition, states that a pure chemical compound always contains the same proportion of elements by mass, regardless of the source of the sample. This principle is fundamental in chemistry, as it allows scientists to determine the composition of compounds based on mass ratios.
To illustrate this concept, consider carbon dioxide (CO2), which consists of one carbon atom and two oxygen atoms. The atomic mass of carbon is approximately 12.01 grams per mole, while oxygen has an atomic mass of about 16 grams per mole. Therefore, the total mass contributed by carbon in CO2 is 12.01 grams, and the total mass contributed by oxygen is 32 grams (2 × 16 grams). The mass ratio is calculated by placing the larger mass on top, resulting in:
Mass Ratio = \(\frac{32 \text{ grams (Oxygen)}}{12.01 \text{ grams (Carbon)}} \approx 2.66\)
This ratio indicates that there are approximately 2.66 grams of oxygen for every gram of carbon in carbon dioxide. According to the law of definite proportions, if two samples of CO2 are taken from different locations, such as New York City and London, they should yield the same mass ratio of 2.66, confirming that both samples are indeed the same compound.
Understanding this law is crucial for performing calculations related to the composition of compounds. By applying the mass ratios derived from the law of definite proportions, chemists can analyze unknown samples and determine their identity based on their elemental composition. This foundational concept not only aids in chemical analysis but also reinforces the consistency and predictability of chemical compounds in nature.