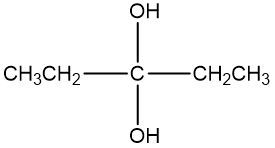
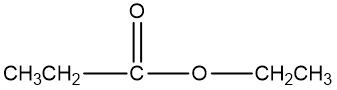
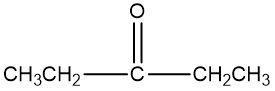
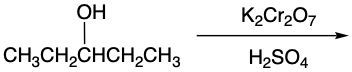In the study of alcohol reactions, particularly oxidation reactions, it is essential to understand the role of oxidizing agents such as Sodium Dichromate or Potassium Dichromate. These agents are interchangeable due to both sodium and potassium being Group 1A elements, but the key component responsible for oxidation is the dichromate ion. When these oxidizing agents are dissolved in sulfuric acid and react with an alcohol, they facilitate the addition of carbon-oxygen bonds while preserving carbon-carbon bonds.
In organic chemistry, the focus of oxidation reactions is primarily on the transformation of alcohols into various functional groups. Initially, an alcohol can be oxidized to form a carbonyl compound, which may manifest as either an aldehyde or a ketone. This step is crucial as it represents the first stage of oxidation. The general reaction can be summarized as:
Alcohol → Aldehyde/Ketone
Furthermore, this carbonyl compound can undergo further oxidation to yield a carboxylic acid. Thus, the complete oxidation pathway can be represented as:
Alcohol → Aldehyde/Ketone → Carboxylic Acid
Throughout this process, it is vital to adhere to the principle of adding as many carbon-oxygen bonds as possible without breaking any carbon-carbon bonds. This rule ensures the correct formation of oxidized products, highlighting the importance of functional group transformations in organic synthesis.