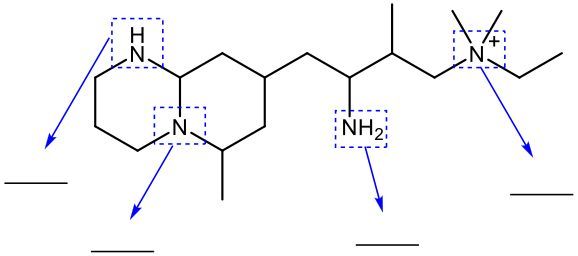
Amines are organic compounds that contain a nitrogen atom bonded to carbon atoms. They are classified based on the number of carbon atoms directly attached to the nitrogen. This classification includes primary, secondary, tertiary, and quaternary amines. A primary amine, denoted as 1°, has one carbon atom bonded to the nitrogen. A secondary amine, or 2°, has two carbon atoms bonded to the nitrogen. A tertiary amine, represented as 3°, has three carbon atoms attached to the nitrogen.
When nitrogen forms four bonds, it transitions from being classified as an amine to an ammonium ion, which is referred to as a quaternary ammonium ion (4°). In this case, the nitrogen atom is bonded to four carbon atoms and carries a formal charge of +1. This positive charge arises because nitrogen, when forming four bonds, has one fewer electron than it needs to achieve a neutral state.
To summarize the classifications: a primary amine has one carbon attached, a secondary amine has two, a tertiary amine has three, and a quaternary ammonium ion has four carbon atoms bonded to the nitrogen. The formal charge of the quaternary ammonium ion is crucial to understanding its behavior in chemical reactions and interactions.