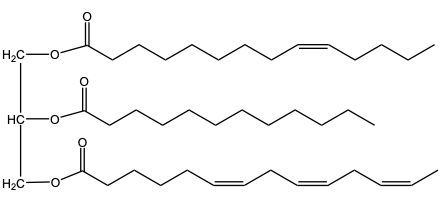
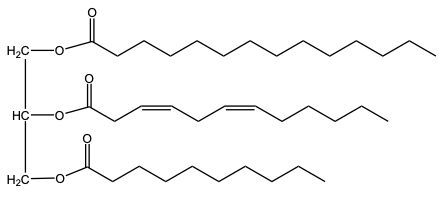
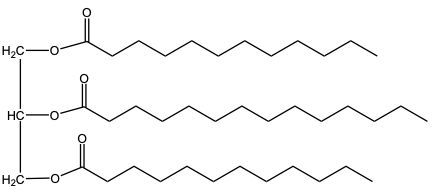
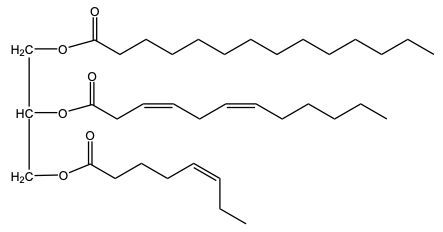
Hydrogenation is a chemical reaction involving the addition of hydrogen (H2) to unsaturated fats, specifically targeting pi bonds in fatty acid chains. This process can lead to the conversion of double bonds into single bonds, effectively decreasing the degree of unsaturation in the fat. As a result, the melting point of the fat increases due to the formation of more stable single bonds.
There are two types of hydrogenation: complete and partial. In complete hydrogenation, all carbon-carbon double bonds in a triglyceride, such as trioline, are fully reduced to single bonds. For every pi bond that is converted, one mole of hydrogen is required. Therefore, if a triglyceride has three double bonds, three moles of H2 are needed for complete hydrogenation, often facilitated by a metal catalyst like nickel.
On the other hand, partial hydrogenation involves the reduction of some, but not all, double bonds. For instance, starting with three double bonds and ending with one means that two pi bonds have been removed, requiring two moles of H2 for the reaction. This method is particularly useful in the food industry, as it allows for the production of various types of margarines with different consistencies. The hardness or softness of margarine is influenced by the number of remaining pi bonds after partial hydrogenation, enabling manufacturers to tailor products to consumer preferences.
In summary, hydrogenation can be complete or partial, each serving distinct purposes in food production and affecting the physical properties of fats. Understanding these processes is crucial for grasping how different types of fats and oils are modified for culinary uses.