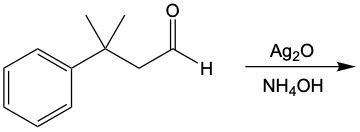
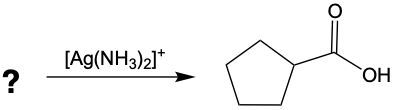
Tollens' test and Benedict's test are both significant oxidation reactions that utilize oxidizing agents to increase the number of carbon-oxygen bonds without disrupting carbon-carbon bonds. Understanding these reactions begins with recognizing the role of alkanes, which can be oxidized to form alcohols. This process involves the addition of oxygen to the carbon framework.
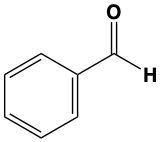
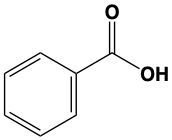
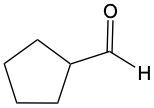
When alcohols undergo oxidation, the hydroxyl group (-OH) on the carbon is transformed into a carbonyl group (C=O), resulting in the formation of aldehydes. This transformation is crucial as it allows for further oxidation. In this step, one of the carbon-hydrogen bonds is broken, and an oxygen atom is introduced, which must form two bonds, typically accompanied by a hydrogen atom. Consequently, the aldehyde is oxidized to a carboxylic acid.
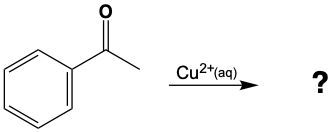
Aldehydes can be oxidized to carboxylic acids, while ketones, which have carbon atoms on both sides of the carbonyl group, do not undergo oxidation in the same manner since breaking carbon-carbon bonds is not permissible. Therefore, the oxidation pathway from an aldehyde to a carboxylic acid is a key focus, illustrating the conversion of functional groups while adhering to the principle of maintaining carbon-carbon bonds intact.
In summary, the oxidation of alcohols to aldehydes and subsequently to carboxylic acids exemplifies the systematic approach to increasing carbon-oxygen bonds through controlled reactions, highlighting the importance of understanding these transformations in organic chemistry.