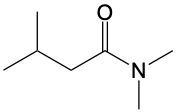
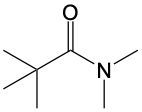
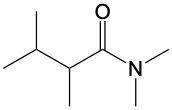
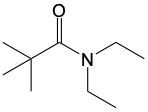
Amide formation is a significant chemical reaction where a carboxylic acid reacts with an amine through a condensation process to produce an amide. In this reaction, water is eliminated, which is a hallmark of condensation reactions. Specifically, the hydroxyl group (–OH) from the carboxylic acid and one hydrogen atom from the amine's nitrogen are removed, resulting in the formation of water.
For the reaction to proceed, the nitrogen atom in the amine must have at least one hydrogen atom available. Additionally, an acid catalyst (H+) is often required to initiate the reaction, facilitating the process.
The general reaction can be represented as follows: a carboxylic acid, denoted as RCOOH, reacts with an amine, R'NH2. During the reaction, the –OH from the carboxylic acid and an H from the amine are lost, forming water (H2O). The carbon atom from the carboxylic acid and the nitrogen atom from the amine then form a new bond, resulting in the creation of an amide, which is characterized by a carbonyl group (C=O) bonded to a nitrogen atom (–NH2).
In summary, the formation of an amide through the condensation of a carboxylic acid and an amine is a crucial reaction in organic chemistry, highlighting the interplay between functional groups and the importance of catalysts in facilitating chemical transformations.