Nucleosides are named based on the nitrogenous base they contain, with specific suffix modifiers indicating their classification. Nitrogenous bases are categorized into two groups: pyrimidines and purines. Pyrimidines, which consist of a single ring structure, have their names modified with the suffix idine, while purines, characterized by a double ring structure, use the suffix osine.
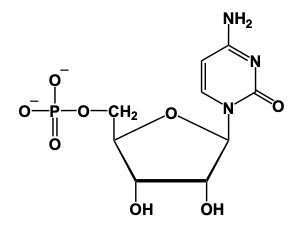
For example, in RNA, the nitrogenous base uracil combines with ribose sugar to form a nucleoside. During this process, a condensation reaction occurs, resulting in the loss of water and the formation of a glycosidic bond between the nitrogen atom of uracil and the anomeric carbon of ribose. Consequently, uracil is renamed to uridine due to the suffix change.
In contrast, DNA utilizes deoxyribose sugar instead of ribose. When adenine, a purine, forms a nucleoside with deoxyribose, a glycosidic bond is also established. Here, it is essential to note the prefix deoxy is added to indicate that the second carbon lacks a hydroxyl group (OH) and instead has a hydrogen (H). Thus, adenine becomes deoxyadenosine, reflecting both the absence of the hydroxyl group and the purine structure.
Understanding these naming conventions is crucial for accurately identifying and differentiating between various nucleosides in both RNA and DNA.