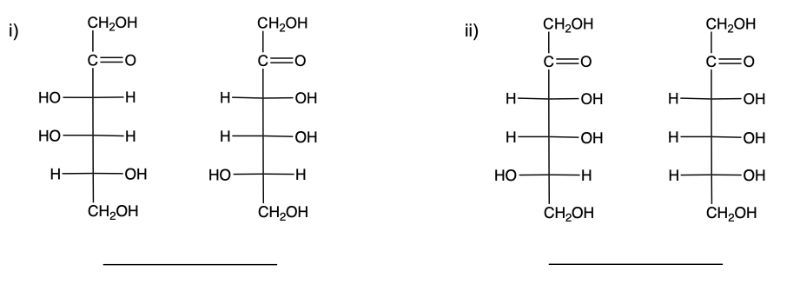
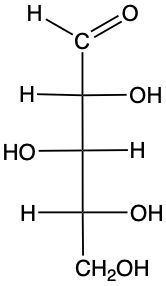
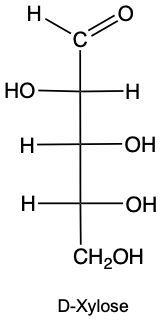
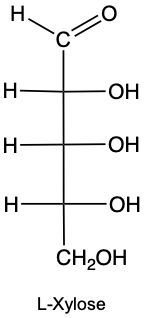
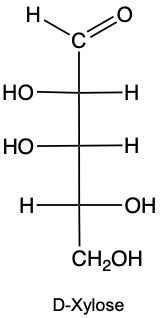
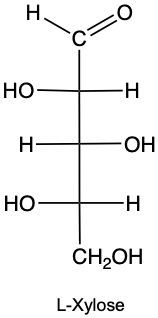
Stereoisomers are compounds that share the same molecular formula and connectivity but differ in their spatial arrangement. Within this category, enantiomers and diastereomers are two important types. Enantiomers are chiral molecules that are non-superimposable mirror images of each other. This means that when comparing two chiral molecules, if they have opposite configurations at all their chiral centers, they are classified as enantiomers.
On the other hand, diastereomers are stereoisomers that are not mirror images. To distinguish diastereomers from enantiomers, one can observe the configurations of their chiral centers. In diastereomers, some chiral centers remain the same while others differ. For example, if two structures have two chiral centers and one chiral center is identical in both while the other is different, these two structures are diastereomers.
To illustrate, consider two structures with two chiral centers. If the hydroxyl (OH) groups are positioned oppositely in a mirror image, they are enantiomers. Conversely, if one chiral center is the same and the other differs, they are diastereomers. This distinction is crucial in stereochemistry, as it affects the properties and reactivity of the compounds.
Additionally, the total number of stereoisomers for a compound can be calculated using the formula \(2^n\), where \(n\) represents the number of chiral centers. For instance, if a structure has two chiral centers, the calculation would be \(2^2\), resulting in four possible stereoisomers. This includes all combinations of configurations for the chiral centers, leading to a total of four distinct structures.
Understanding the differences between enantiomers and diastereomers, as well as how to calculate the number of stereoisomers, is essential for grasping the complexities of stereochemistry and its implications in various chemical reactions and applications.