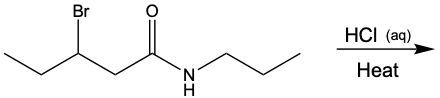
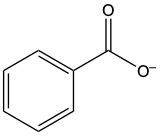
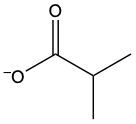
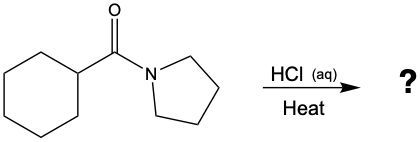
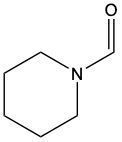
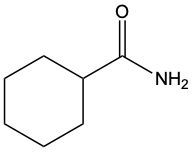
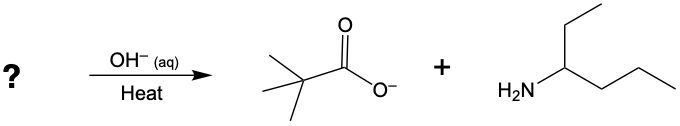
Acidic hydrolysis of amides is a chemical reaction where an amide is converted into a carboxylic acid and an ammonium ion in the presence of an acidic medium. In this process, the amide bond, which connects the nitrogen atom to the carbonyl group, is broken. The reaction typically involves aqueous hydrochloric acid and requires heat to proceed effectively.
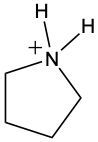
During the reaction, the carbonyl carbon of the amide gains a hydroxyl group (–OH), transforming it into a carboxylic acid. Simultaneously, the nitrogen atom in the amide gains two hydrogen atoms, resulting in the formation of an ammonium ion. This change occurs because when nitrogen forms four bonds, it carries a positive charge, transitioning from a neutral amine to an ammonium ion.
In summary, the key products of acidic hydrolysis of amides are a carboxylic acid and an ammonium ion, highlighting the transformation of the amide structure under acidic conditions.