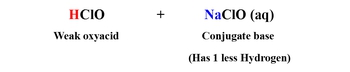Buffer solutions are essential in maintaining stable pH levels in various chemical environments. A buffer typically consists of a weak acid and its conjugate base. For example, hydrofluoric acid (HF) is a weak acid, and its conjugate base can be represented as sodium fluoride (NaF), which is formed by removing one hydrogen ion (H+) from HF, resulting in fluoride ion (F-). This combination allows the buffer to resist significant changes in pH when small amounts of strong acids or bases are added.
The mechanism behind a buffer's effectiveness lies in its ability to maintain constant concentrations of hydrogen ions (H+) and hydroxide ions (OH-). When a strong base is introduced, the pH tends to increase; however, the weak acid in the buffer neutralizes the added base, preventing a drastic rise in pH. Conversely, if a strong acid is added, the conjugate base reacts with the acid, minimizing the decrease in pH. This interplay between the weak acid and its conjugate base is crucial, as they act as natural antagonists to any added acids or bases.
It is important to note that buffers can be compromised if excessive amounts of strong acids or bases are introduced. In contrast, adding water to a buffer solution does not alter its effectiveness. This is because the addition of water dilutes both the weak acid and the conjugate base proportionally, maintaining the necessary ratio for buffering capacity.
To identify whether a combination of substances forms a buffer, one must recognize the characteristics of weak acids and their conjugate bases. Understanding these principles will aid in determining which combinations do not create a buffer solution. Engaging with practice questions can further solidify this knowledge and enhance your ability to apply these concepts effectively.