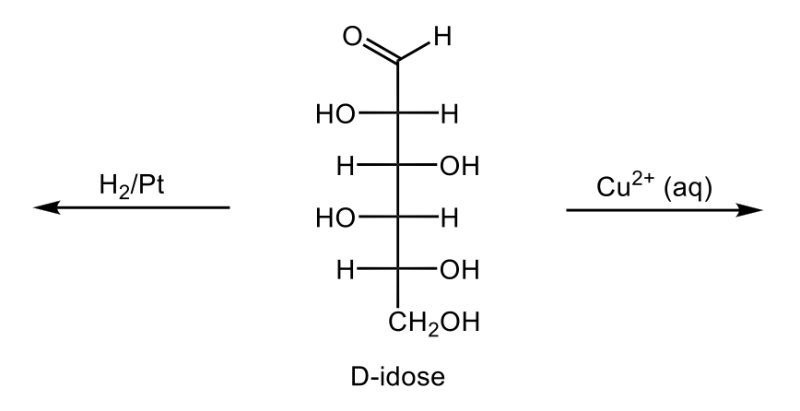
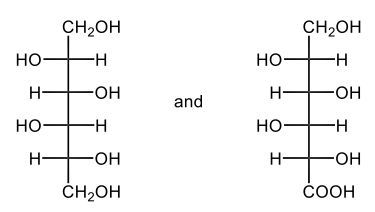
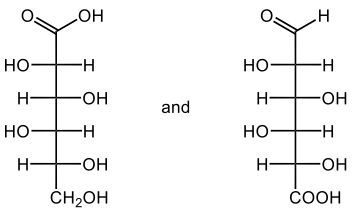
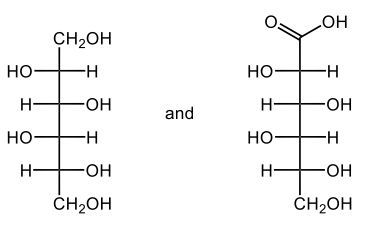
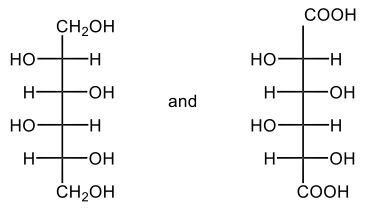The oxidation of monosaccharides is a significant chemical process, particularly illustrated by the Benedict's test. In this test, an aldehyde group present in aldose sugars undergoes oxidation, transforming into a carboxylic acid and resulting in the formation of a brick red precipitate. This transformation is crucial as it leads to the production of what are known as aldonic acids, which are essentially sugar acids. The nomenclature changes from 'ose' to 'onic acid' to reflect this conversion.
For example, consider D-ribose, a typical aldose sugar characterized by its aldehyde group. When D-ribose is oxidized using copper(II) ions, it converts into ribonic acid, maintaining its classification as a D-sugar. The oxidation introduces a carboxylic acid group, and the resulting byproduct of this reaction is copper(I) oxide, which appears as the solid brick red precipitate.
Furthermore, the concept of reducing sugars is integral to this discussion. A reducing sugar is defined as a carbohydrate that can be oxidized to produce a sugar acid, such as D-ribose. The Benedict's test serves as a practical method for detecting reducing sugars in solution, highlighting the importance of this oxidation process in carbohydrate chemistry. In summary, the oxidation of aldose sugars not only alters their chemical structure but also provides a means to identify them through tests like Benedict's, emphasizing the transition from aldose sugars to aldonic acids.