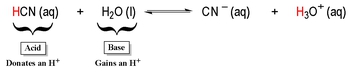
In 1923, Bronsted and Lowry introduced a significant advancement in the understanding of acids and bases, refining earlier definitions established by Arrhenius. According to the Bronsted-Lowry theory, an acid is defined as a proton donor, specifically referring to the hydrogen ion (H+). This aligns with the Arrhenius definition, which states that an Arrhenius acid increases the concentration of H+ ions in aqueous solutions.
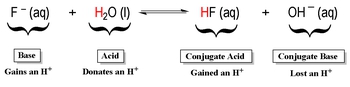
However, the Bronsted-Lowry theory diverges from Arrhenius in its definition of bases. While Arrhenius defined a base as a substance that produces hydroxide ions (OH-) in water, Bronsted and Lowry proposed that a base is a proton acceptor. This means that a base can accept H+ ions, which is often facilitated by the presence of lone pairs of electrons or a negative charge. This conceptual shift allows for the classification of acids and bases in non-aqueous solutions, broadening the scope of acid-base chemistry.
Both theories agree on the behavior of acids: Bronsted-Lowry acids donate H+ ions, which is consistent with the Arrhenius definition. However, the Bronsted-Lowry definition emphasizes the interaction between acids and bases, highlighting that they always exist in pairs known as conjugate acid-base pairs. These pairs differ by a single hydrogen ion. For example, water (H2O) can act as both an acid and a base, forming hydroxide ions (OH-) and hydronium ions (H3O+), respectively. In this case, H2O and OH- are conjugate acid-base pairs, differing by one H+ ion.
Understanding these concepts is crucial for grasping the dynamics of acid-base reactions and their applications in various chemical contexts.