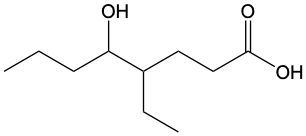
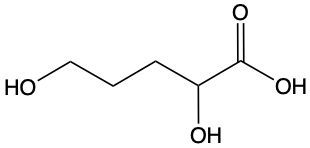
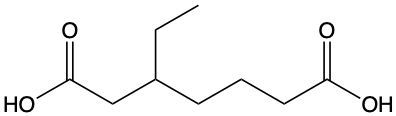
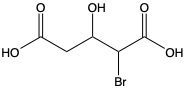
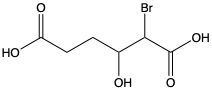
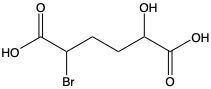
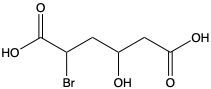
Carboxylic acids are organic compounds characterized by the presence of a carbonyl group (C=O) directly bonded to a hydroxyl group (–OH). This functional group structure is essential for their chemical properties and reactivity. When naming carboxylic acids, the rules are similar to those for aldehydes, primarily because the carbonyl carbon is always assigned the number 1 position in the carbon chain.
To name a carboxylic acid, the suffix of the corresponding alkane is modified from an 'e' to 'oic acid'. For example, the alkane "pentane" becomes "pentanoic acid" when it is converted into a carboxylic acid. Additionally, it is important to indicate the positions of any substituents on the carbon chain, ensuring clarity in the compound's structure. This systematic approach to naming helps in identifying the specific carboxylic acid and its derivatives accurately.