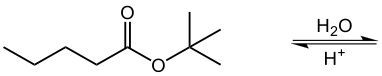
In the process of acid-catalyzed hydrolysis of esters, an ester undergoes a reaction with water in the presence of an acid catalyst, typically represented as H+. This reaction effectively breaks the ester bond, resulting in the formation of a carboxylic acid and an alcohol. Hydrolysis, in this context, refers to the chemical breakdown of a compound due to its reaction with water.
To illustrate, consider the general reaction where the ester bond is cleaved. The carbonyl carbon of the ester gains a hydroxyl group (–OH), transforming it into a carboxylic acid. Simultaneously, the oxygen atom that was part of the ester linkage acquires a hydrogen atom, resulting in the formation of an alcohol. Thus, the overall reaction can be summarized as:
Esters + H2O (in the presence of H+) → Carboxylic Acid + Alcohol
This reaction highlights the importance of acid catalysis in facilitating the hydrolysis process, allowing for the efficient conversion of esters into valuable products such as carboxylic acids and alcohols.