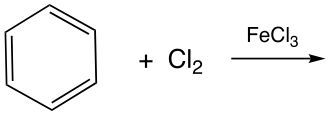Benzene is classified as an aromatic compound, which is characterized by its unique stability due to the presence of delocalized π (pi) electrons. This stability, known as aromaticity, allows benzene to resist typical reactions that alkenes and alkynes undergo, such as addition reactions. Instead, benzene primarily participates in substitution reactions, which help preserve its stable structure.
Two significant types of substitution reactions that benzene undergoes are halogenation and Friedel-Crafts alkylation. In halogenation, a halogen atom is substituted onto the benzene ring, replacing one of the hydrogen atoms. This reaction typically requires a halogen source and a catalyst to facilitate the process.
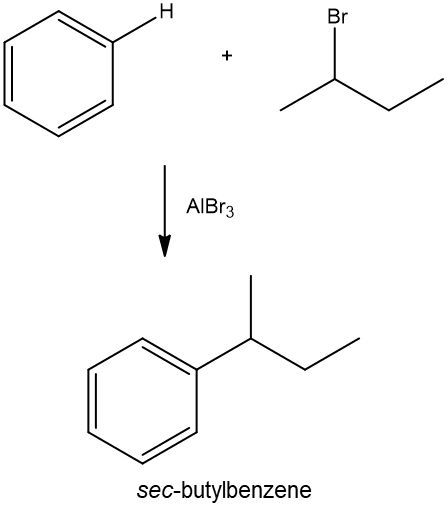
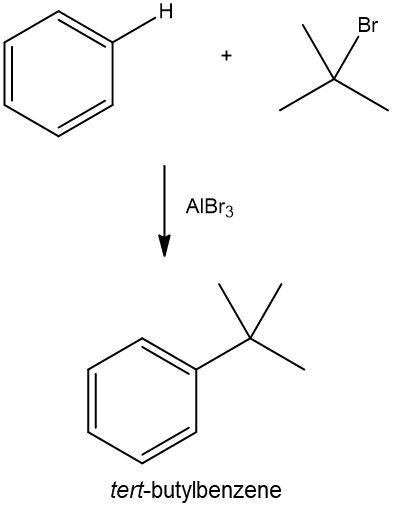
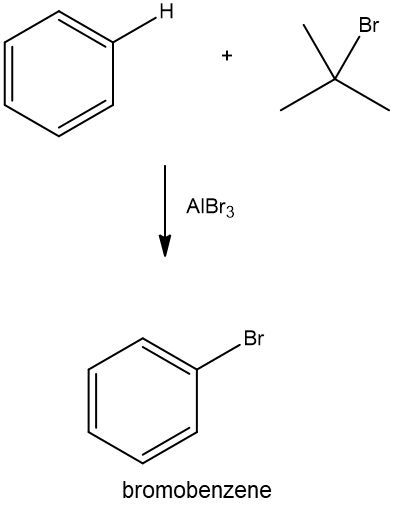
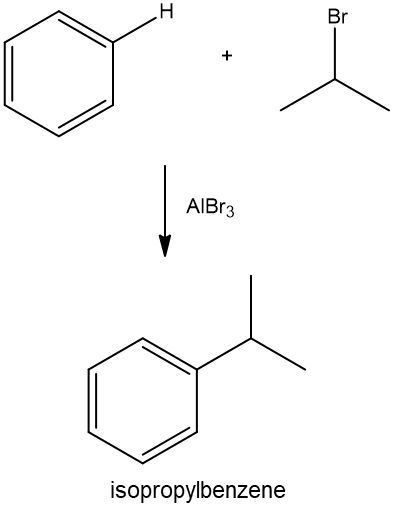
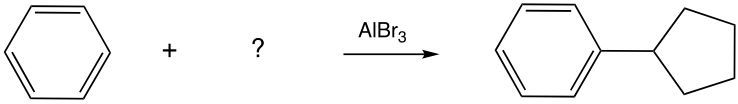
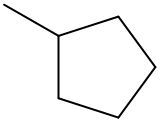
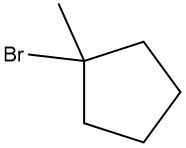
Friedel-Crafts alkylation involves the substitution of an alkyl group onto the benzene ring. This reaction also requires a catalyst, often a Lewis acid, to activate the alkyl halide, allowing it to bond with the benzene. Both of these reactions exemplify how benzene maintains its aromatic stability while allowing for functional group modifications.
In summary, the unique stability of benzene as an aromatic compound leads to its preference for substitution reactions over addition reactions, ensuring that its aromaticity is preserved throughout chemical transformations.