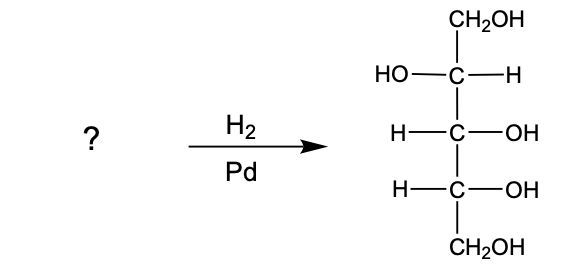
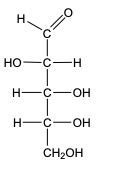
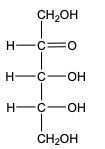
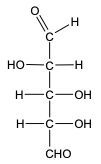
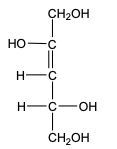
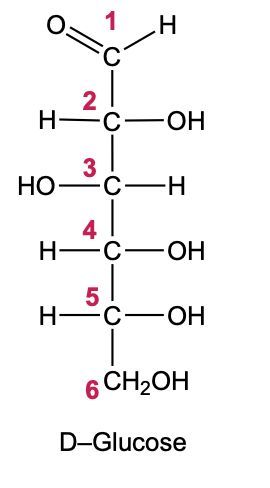
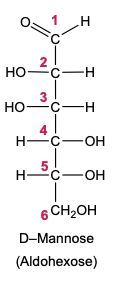
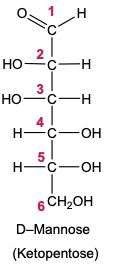
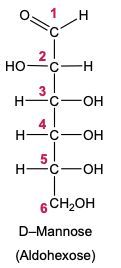
In the reduction of aldose or keto sugars, the carbonyl group undergoes a transformation into a hydroxyl group, resulting in the formation of a sugar alcohol. A sugar alcohol is defined as a monosaccharide where each carbon atom is bonded to a hydroxyl group (–OH). The reduction process involves a reducing agent, typically hydrogen gas (H2), and is facilitated by a metal catalyst such as nickel, platinum, or palladium.
During this reaction, the oxygen atom of the carbonyl group receives a hydrogen atom, while the carbon atom of the carbonyl group also gains a hydrogen atom. This dual addition of hydrogen atoms effectively converts the carbonyl functional group into a hydroxyl group, leading to the synthesis of a sugar alcohol from the original aldose or keto sugar.