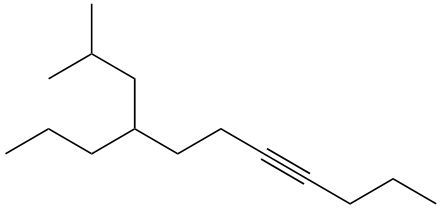
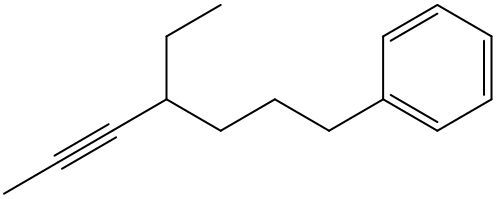
Alkynes are hydrocarbons characterized by the presence of a carbon-carbon triple bond, which significantly influences their chemical properties and nomenclature. The naming conventions for alkynes closely resemble those for alkenes, with a key distinction in the suffix used. Instead of the suffix -ane found in alkanes, alkynes utilize -yne to indicate the presence of the triple bond.
One important aspect of alkynes is that they do not exhibit cis or trans isomerism due to the linear geometry around the triple bond. When naming alkynes, it is essential to specify the location of the triple bond and any substituents present in the molecule. This is done by assigning numerical locants to both the triple bond and the substituents, ensuring clarity in the molecular structure.
For example, in a compound with a triple bond starting at the first carbon, the name would reflect this position, such as in 1-butyne for a four-carbon chain with a triple bond at the first carbon. Understanding these naming conventions is crucial for accurately identifying and communicating the structure of alkynes in organic chemistry.