Skeletal formulas, also known as bond line or line formulas, serve as an efficient method for representing complex organic structures in chemistry. As one progresses in the study of organic chemistry, the reliance on structural and condensed formulas diminishes due to their time-consuming nature. Instead, skeletal formulas become the preferred choice for illustrating intricate organic compounds.
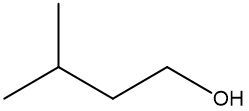
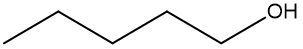
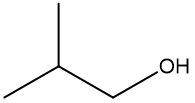
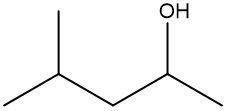
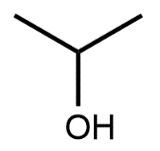
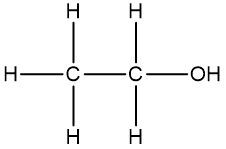
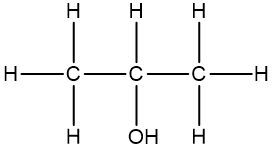
In a skeletal formula, carbon-carbon bonds are depicted as lines, with each corner of the line representing a carbon atom. Notably, hydrogen atoms attached to carbon are typically omitted for simplicity, while other atoms such as oxygen, nitrogen, and sulfur are explicitly shown. For instance, in a skeletal representation of a compound like CH3CH2OH, the carbon atoms are implied at the corners, and the oxygen atom is clearly indicated.
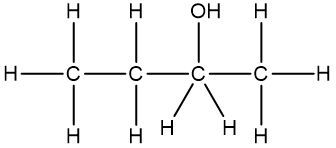
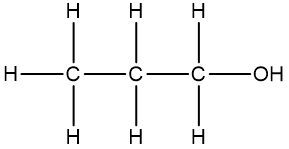
It is essential to remember that carbon atoms must form four bonds. In a skeletal formula, if a carbon atom is connected to another carbon, the remaining bonds are filled with invisible hydrogen atoms. For example, if a carbon atom is bonded to another carbon and has three hydrogen atoms, those hydrogens are not drawn but are understood to be present. Similarly, if a carbon is bonded to an oxygen, any hydrogen atoms attached to the oxygen are shown, while those attached to carbon are not.
Overall, skeletal formulas provide a streamlined way to represent both condensed and structural formulas, making them invaluable as one delves deeper into the complexities of organic chemistry.