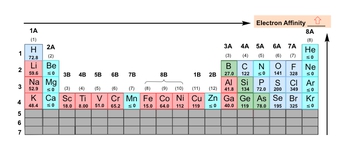
Electron affinity, denoted as \( e_a \), quantifies the energy released when an electron is added to a gaseous atom or ion, measured in kilojoules. For instance, when considering gaseous carbon, the process involves adding an electron, which transforms the neutral carbon atom into a negatively charged ion. This addition of an electron is a reactant in the reaction, while the energy released during this process is the electron affinity, making it a product.
However, there are exceptions in the realm of electron affinity. Some elements exhibit electron affinities that are less than or equal to zero, indicating a reluctance to accept an additional electron. This behavior is often observed in elements with stable electron configurations, such as the noble gases. These gases possess a complete valence shell, rendering them "perfect" in terms of electron arrangement, and thus they do not have a tendency to gain more electrons.
In terms of periodic trends, electron affinity generally increases as one moves from left to right across a period and decreases as one moves down a group in the periodic table. A lower electron affinity suggests that an element is less inclined to accept an electron, while a higher electron affinity indicates a greater likelihood of accepting an electron. Therefore, understanding electron affinity is crucial for predicting the reactivity and behavior of elements in chemical reactions.