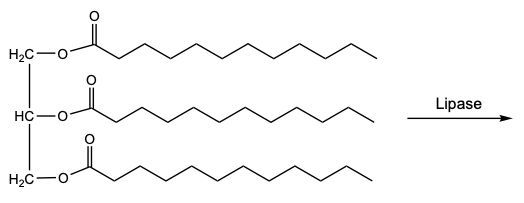
Triacylglycerol hydrolysis is a crucial biochemical reaction that can occur through two primary methods: acid-catalyzed hydrolysis and enzymatic hydrolysis. In acid-catalyzed hydrolysis, strong acids such as hydrochloric acid (HCl) or sulfuric acid (H₂SO₄) facilitate the breakdown of ester bonds in triacylglycerols. This process occurs stepwise, meaning that the three fatty acids are released one at a time rather than all at once. Initially, the first ester linkage is hydrolyzed, releasing the first fatty acid, followed by the second and third in subsequent steps.
During this reaction, water (H₂O) plays a vital role in cleaving the ester bonds. The addition of water results in the formation of hydroxyl (OH) groups on the glycerol molecule, which is composed of a glycerol backbone and three fatty acid chains. Each fatty acid consists of a hydrocarbon tail and a carboxylic acid head, which is formed by adding an OH group to the carbonyl carbon during hydrolysis. The overall reaction can be summarized as:
Triacylglycerol + 3 H₂O → Glycerol + 3 Fatty Acids
In contrast, enzymatic hydrolysis employs the digestive enzyme lipase under milder conditions to achieve the same end products: one glycerol molecule and three fatty acids. Although the mechanisms differ, both methods ultimately yield the same products, highlighting the versatility of biochemical pathways in lipid metabolism.