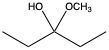
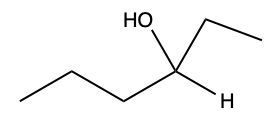
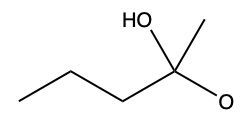
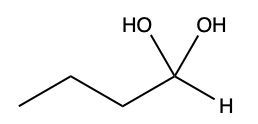
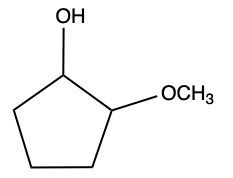
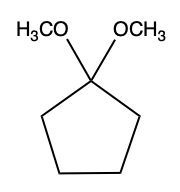
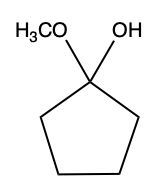
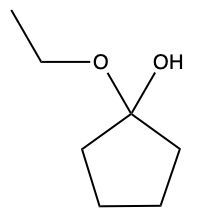
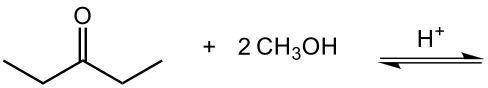Aldehydes and ketones undergo reactions with alcohols, resulting in the formation of hemiacetals and acetals. A hemiacetal is defined as a compound that contains both a hydroxy group (–OH) and an alkoxy group (–OR) attached to the same carbon atom. In this context, the symbol R serves as a placeholder for any carbon-containing group, which could range from a simple methyl or ethyl group to more complex structures like rings or long carbon chains.
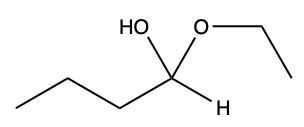
When an aldehyde reacts with an alcohol, the product is a carbon atom bonded to both an –OH group and an –OR group, thus forming a hemiacetal. This reaction can be represented as follows:
RCHO + R'OH → RCH(OH)OR'
Here, RCHO represents the aldehyde, and R'OH represents the alcohol. The resulting compound, RCH(OH)OR', is the hemiacetal.
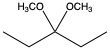
To convert a hemiacetal into a full acetal, the hemiacetal can react with an additional mole of alcohol. In this reaction, the hydroxy group (–OH) is replaced by another alkoxy group (–OR), leading to a compound with two alkoxy groups on the same carbon atom. This final product is known as an acetal, which can be represented as:
RCH(OR')2
In summary, the reaction pathway begins with aldehydes or ketones reacting with one mole of alcohol to form a hemiacetal. This hemiacetal can further react with another mole of alcohol to yield a full acetal. Understanding these transformations is crucial in organic chemistry, particularly in the study of carbohydrate chemistry and synthesis.