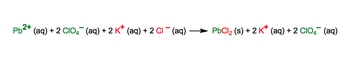
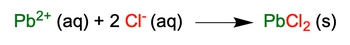In chemistry, understanding the transition from a molecular equation to a complete ionic equation is crucial for analyzing reactions in aqueous solutions. A molecular equation presents all reactants and products in their molecular forms, including solids, liquids, and gases, while a complete ionic equation breaks down only the aqueous compounds into their constituent ions.
When dealing with complete ionic equations, it is essential to recognize that only aqueous compounds dissociate into ions. Solids, liquids, and gases remain intact and do not break apart. To determine whether a compound is aqueous, solubility rules are applied, which help identify which substances will dissolve in water and form ions.
To derive a complete ionic equation from a molecular equation, one must distribute the coefficients of each compound correctly. This ensures that the number of ions represented reflects the actual quantities involved in the reaction. For example, if a molecular equation shows a compound with a coefficient of 2, this indicates that two moles of that compound will dissociate into ions, effectively doubling the number of ions in the complete ionic equation.
In summary, the complete ionic equation provides a clearer picture of the chemical processes occurring in solution by illustrating how aqueous compounds dissociate into ions, while maintaining the integrity of solids, liquids, and gases. This understanding is fundamental for predicting the outcomes of chemical reactions and for further studies in solution chemistry.