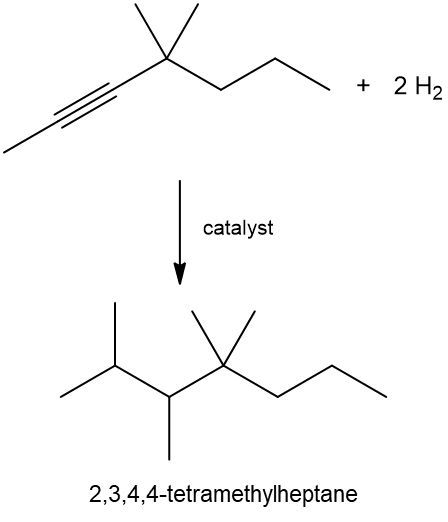
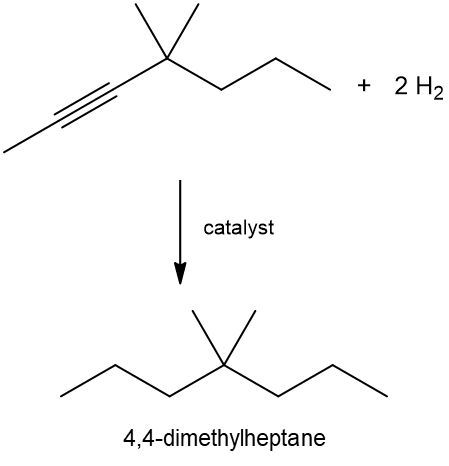
Hydrogenation reactions involve the addition of hydrogen (H2) to unsaturated hydrocarbons, specifically alkenes and alkynes, resulting in the formation of alkanes. In these reactions, the addition of hydrogen occurs across pi bonds, which are present in unsaturated compounds. For an alkene, which contains one pi bond, one mole of H2 is required. A catalyst, typically a metal, is necessary to facilitate the breaking of the H-H bond due to the stability of molecular hydrogen.
When hydrogen is added to an alkene, the pi bond is broken, allowing the two hydrogen atoms to bond with the two carbon atoms that were previously double-bonded. This process transforms the alkene into an alkane, characterized by single bonds between carbon and hydrogen atoms.
In the case of alkynes, which contain two pi bonds, the hydrogenation process requires two moles of H2. Each pi bond requires one mole of hydrogen, leading to the addition of four hydrogen atoms in total. Regardless of whether the starting material is an alkene or an alkyne, the end product of a hydrogenation reaction is always an alkane, which is fully saturated with single bonds.