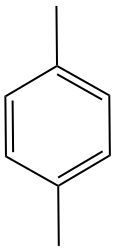
Monosubstituted benzenes are organic compounds where a benzene ring serves as the parent structure, featuring only one substituent. This simplicity in structure allows for straightforward naming conventions. When naming a monosubstituted benzene, the name of the substituent is placed before the term "benzene." For example, if the substituent is a methyl group, the compound is named "toluene." Since there is only one substituent, specifying its position on the benzene ring is unnecessary, making the naming process more efficient. Understanding this basic naming convention is essential for further studies in organic chemistry, particularly in the context of aromatic compounds.
- 1. Matter and Measurements4h 29m
- What is Chemistry?7m
- The Scientific Method9m
- Classification of Matter16m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature (Simplified)9m
- Scientific Notation13m
- SI Units (Simplified)5m
- Metric Prefixes24m
- Significant Figures (Simplified)11m
- Significant Figures: Precision in Measurements7m
- Significant Figures: In Calculations19m
- Conversion Factors (Simplified)15m
- Dimensional Analysis22m
- Density12m
- Specific Gravity9m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects8m
- 2. Atoms and the Periodic Table5h 22m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)18m
- Atomic Mass (Conceptual)12m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Elemental Forms (Simplified)6m
- Periodic Table: Phases (Simplified)8m
- Law of Definite Proportions9m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)22m
- Electron Arrangements5m
- The Electron Configuration: Condensed4m
- The Electron Configuration: Exceptions (Simplified)12m
- Ions and the Octet Rule9m
- Ions and the Octet Rule (Simplified)8m
- Valence Electrons of Elements (Simplified)5m
- Lewis Dot Symbols (Simplified)7m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- 3. Ionic Compounds2h 18m
- Periodic Table: Main Group Element Charges12m
- Periodic Table: Transition Metal Charges5m
- Periodic Trend: Ionic Radius (Simplified)5m
- Periodic Trend: Ranking Ionic Radii8m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)8m
- Ionic Bonding6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- 4. Molecular Compounds2h 18m
- Covalent Bonds6m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Bonding Preferences6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Multiple Bonds4m
- Multiple Bonds (Simplified)6m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)8m
- Molecular Geometry (Simplified)11m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)15m
- Molecular Polarity (Simplified)7m
- 5. Classification & Balancing of Chemical Reactions3h 17m
- Chemical Reaction: Chemical Change5m
- Law of Conservation of Mass5m
- Balancing Chemical Equations (Simplified)13m
- Solubility Rules16m
- Molecular Equations18m
- Types of Chemical Reactions12m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Spontaneous Redox Reactions8m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Balancing Redox Reactions (Simplified)13m
- Galvanic Cell (Simplified)16m
- 6. Chemical Reactions & Quantities2h 34m
- 7. Energy, Rate and Equilibrium3h 40m
- Nature of Energy6m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Bond Energy14m
- Thermochemical Equations12m
- Heat Capacity19m
- Thermal Equilibrium (Simplified)8m
- Hess's Law23m
- Rate of Reaction11m
- Energy Diagrams12m
- Chemical Equilibrium7m
- The Equilibrium Constant14m
- Le Chatelier's Principle20m
- Solubility Product Constant (Ksp)17m
- Spontaneous vs Nonspontaneous Reactions7m
- Entropy (Simplified)9m
- Gibbs Free Energy (Simplified)18m
- 8. Gases, Liquids and Solids3h 27m
- Pressure Units6m
- Kinetic Molecular Theory14m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws17m
- Chemistry Gas Laws: Combined Gas Law12m
- Standard Temperature and Pressure14m
- Dalton's Law: Partial Pressure (Simplified)13m
- Gas Stoichiometry18m
- Intermolecular Forces (Simplified)19m
- Intermolecular Forces and Physical Properties11m
- Atomic, Ionic and Molecular Solids10m
- Heating and Cooling Curves30m
- 9. Solutions4h 27m
- Solutions6m
- Solubility and Intermolecular Forces17m
- Solutions: Mass Percent6m
- Percent Concentrations10m
- Molarity18m
- Osmolarity15m
- Parts per Million (ppm)13m
- Solubility: Temperature Effect8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- Dilutions12m
- Solution Stoichiometry14m
- Electrolytes (Simplified)13m
- Equivalents11m
- Molality15m
- The Colligative Properties15m
- Boiling Point Elevation16m
- Freezing Point Depression9m
- Osmosis16m
- Osmotic Pressure10m
- Vapor Pressure Lowering (Raoult's Law)16m
- 10. Acids and Bases3h 10m
- Acid-Base Introduction11m
- Arrhenius Acid and Base6m
- Bronsted Lowry Acid and Base21m
- Acid and Base Strength17m
- Ka and Kb16m
- The pH Scale16m
- Auto-Ionization9m
- pH of Strong Acids and Bases9m
- Acid-Base Equivalents14m
- Acid-Base Reactions7m
- Gas Evolution Equations (Simplified)6m
- Ionic Salts (Simplified)11m
- Buffers11m
- Henderson-Hasselbalch Equation16m
- Strong Acid Strong Base Titrations (Simplified)13m
- 11. Nuclear Chemistry1h 1m
- BONUS: Lab Techniques and Procedures1h 38m
- BONUS: Mathematical Operations and Functions47m
- 12. Introduction to Organic Chemistry1h 34m
- 13. Alkenes, Alkynes, and Aromatic Compounds2h 12m
- 14. Compounds with Oxygen or Sulfur1h 6m
- 15. Aldehydes and Ketones1h 1m
- 16. Carboxylic Acids and Their Derivatives1h 11m
- 17. Amines40m
- 18. Amino Acids and Proteins1h 51m
- 19. Enzymes1h 37m
- 20. Carbohydrates1h 46m
- Intro to Carbohydrates4m
- Classification of Carbohydrates4m
- Fischer Projections4m
- Enantiomers vs Diastereomers7m
- D vs L Enantiomers9m
- Cyclic Hemiacetals8m
- Intro to Haworth Projections4m
- Cyclic Structures of Monosaccharides11m
- Mutarotation4m
- Reduction of Monosaccharides10m
- Oxidation of Monosaccharides7m
- Glycosidic Linkage14m
- Disaccharides7m
- Polysaccharides8m
- 21. The Generation of Biochemical Energy2h 9m
- 22. Carbohydrate Metabolism2h 23m
- 23. Lipids2h 26m
- Intro to Lipids6m
- Fatty Acids25m
- Physical Properties of Fatty Acids6m
- Waxes4m
- Triacylglycerols12m
- Triacylglycerol Reactions: Hydrogenation8m
- Triacylglycerol Reactions: Hydrolysis13m
- Triacylglycerol Reactions: Oxidation7m
- Glycerophospholipids15m
- Sphingomyelins13m
- Steroids15m
- Cell Membranes7m
- Membrane Transport10m
- 24. Lipid Metabolism1h 45m
- 25. Protein and Amino Acid Metabolism1h 37m
- 26. Nucleic Acids and Protein Synthesis2h 54m
- Intro to Nucleic Acids4m
- Nitrogenous Bases16m
- Nucleoside and Nucleotide Formation9m
- Naming Nucleosides and Nucleotides13m
- Phosphodiester Bond Formation7m
- Primary Structure of Nucleic Acids11m
- Base Pairing10m
- DNA Double Helix6m
- Intro to DNA Replication20m
- Steps of DNA Replication11m
- Types of RNA10m
- Overview of Protein Synthesis4m
- Transcription: mRNA Synthesis9m
- Processing of pre-mRNA5m
- The Genetic Code6m
- Introduction to Translation7m
- Translation: Protein Synthesis18m
Naming Benzene: Videos & Practice Problems
Monosubstituted benzenes are named by attaching the substituent name to "benzene," with no locant needed due to a single substituent. Disubstituted benzenes require numbering substituents alphabetically or using ortho (1,2), meta (1,3), and para (1,4) prefixes, aiding in identifying relative positions. Common names like phenol, aniline, and toluene are used for specific substituents. Polysubstituted benzenes use numbering starting at the substituent with the common name, ensuring the lowest locants for others. For example, 2,4,6-trinitrotoluene (TNT) illustrates naming with multiple nitro groups on a methyl-substituted benzene, emphasizing functional group identification and systematic nomenclature.
Monosubstituted Benzene Concept 1
Monosubstituted Benzene Concept 1 Video Summary

Monosubstituted Benzene Example 1
Monosubstituted Benzene Example 1 Video Summary
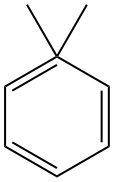
In organic chemistry, naming compounds systematically is essential for clear communication. When analyzing a compound with a benzene ring and an alkyl group, it is important to identify the structure of the alkyl group attached to the benzene. In this case, the alkyl group consists of four carbon atoms, which can be classified as butyl, isobutyl, sec-butyl, or tert-butyl.
To determine the correct name, we examine the structure of the alkyl group. If the connection occurs at a carbon that is bonded to three other methyl groups, this indicates that the alkyl group is a tert-butyl group. The systematic name for this alkyl group is tert-butyl, which is written with a hyphen to separate it from the benzene component.
Thus, when the tert-butyl group is connected to the benzene ring, the full systematic name of the compound becomes tert-butylbenzene. This naming convention follows the IUPAC guidelines, ensuring that the compound is accurately identified in chemical literature.
Disubstituted Benzene Concept 2
Disubstituted Benzene Concept 2 Video Summary
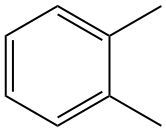
Disubstituted benzenes are aromatic compounds where a benzene ring serves as the parent structure, featuring two substituents. The naming of these compounds follows a systematic approach where substituents are numbered based on alphabetical order. For instance, if the substituents are fluorine and bromine, bromine (bromo) is prioritized, and the numbering begins at its position.
In naming, the locations of each substituent are indicated numerically, followed by the term "benzene." Benzene is distinctive because the positions of the substituents can be described using numerical designations (1,2; 1,3; or 1,4) or through the terms ortho, meta, and para. For example, in dichlorobenzenes, if the chlorines are at positions 1 and 2, it can be referred to as 1,2-dichlorobenzene or ortho-dichlorobenzene. If they occupy positions 1 and 3, it is termed 1,3-dichlorobenzene or meta-dichlorobenzene. Lastly, if they are at positions 1 and 4, it is called 1,4-dichlorobenzene or para-dichlorobenzene.
It is important to note that the two substituents can be identical or different, and the ortho, meta, and para nomenclature remains applicable in both cases. A helpful mnemonic to remember the order of these terms is "Order More Pizza," which corresponds to the positions: 1,2 (ortho), 1,3 (meta), and 1,4 (para). This aids in recalling the naming conventions for disubstituted benzenes effectively.
Disubstituted Benzene Example 2
Disubstituted Benzene Example 2 Video Summary
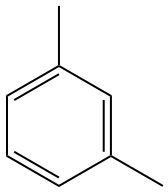
To systematically name a compound featuring a benzene ring connected to two identical ethyl groups, we begin by identifying the structure. The benzene ring serves as the core, and the ethyl groups are two-carbon alkyl chains attached to it. Since both ethyl groups are located at the same positions on the benzene ring, we can number the carbon atoms starting from any carbon, leading to the same numerical designations.
In this case, the ethyl groups occupy the 1 and 3 positions on the benzene ring, which can be denoted as 1,3. Since there are two ethyl groups, we use the prefix "di" to indicate their presence. The systematic name of the compound is therefore 1,3-Diethylbenzene.
If we were to consider a common naming convention using the terms ortho, meta, or para, we would refer to the arrangement of the substituents. In this instance, with the ethyl groups at the 1 and 3 positions, the compound can also be referred to as meta-diethylbenzene. However, the systematic name remains the official designation.
Determine the systematic name of the molecule.
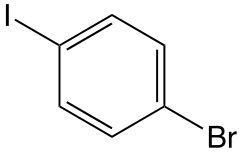
1-iodo-4-bromobenzene
1-bromo-4-iodobenzene
4-bromo-1-iodobenzene
4-iodo-1-bromobenzene
Determine the systematic name of the molecule.
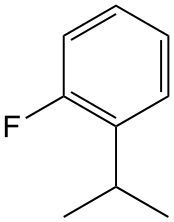
ortho-fluoroisopropylbenzene
para-fluoroisopropylbenzene
meta-fluoroisopropylbenzene
1-fluoro-2-tert-butylbenzene
Common Naming of Disubstituted Benzene Concept 3
Common Naming of Disubstituted Benzene Concept 3 Video Summary
Monosubstituted benzenes have both common and systematic names that are widely recognized in organic chemistry. For instance, when a hydroxyl group (–OH) is attached to benzene, it is referred to as phenol. An amine group (–NH2) leads to the common name aniline, while a methyl group (–CH3) results in toluene. If a methoxy group (–OCH3) is present, the compound is known as anisole. An aldehyde group (–CHO) connected to benzene gives rise to benzaldehyde, and a carboxylic acid group (–COOH) results in benzoic acid.
When considering disubstituted benzenes, where two substituents are present, the common name of one substituent can become the parent name. The relative positions of the substituents are indicated by the prefixes ortho, meta, and para. For example, if a methyl group is attached to benzene, resulting in toluene, and a chlorine atom is also attached at position 2 (with position 1 being the methyl group), this arrangement is referred to as ortho due to the proximity of the two substituents. Therefore, the complete name for this compound would be ortho-chlorotoluene.
Common Naming of Disubstituted Benzene Example 3
Common Naming of Disubstituted Benzene Example 3 Video Summary
To name the given molecule, we start by identifying the key components of its structure. The molecule features a benzene ring with a hydroxyl group (–OH) and a bromine atom (–Br) as substituents. The hydroxyl group, when attached to a benzene ring, is commonly referred to as phenol.
Next, we assign the name to the bromine substituent, which is denoted as bromo. To indicate the positions of the substituents on the benzene ring, we number the carbon atoms starting from the carbon bonded to the hydroxyl group, which is designated as carbon number 1. The subsequent carbons are numbered as 2, 3, and 4. In this case, the bromine is located at carbon 4, making the relationship between the two substituents a para configuration.
Finally, we combine these elements to form the name of the compound. The complete name, written without spaces, is para-bromophenol. Alternatively, it can be abbreviated as p-bromophenol, where 'p' stands for para. This naming convention effectively communicates the structure of this disubstituted benzene compound.
Write a common name for the following compound.
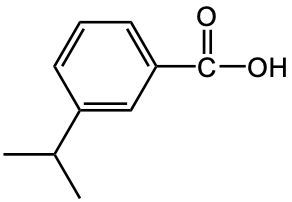
o-tertbutylbenzoic acid
p-isopropylbenzoic acid
5-tertbytylbenzoic acid
m-isopropylbenzoic acid
The common name for a disbstituted benzene with two methyl groups is xylene. Draw a structure for meta-xylene.
Naming Polysubstituted Benzene Concept 4
Naming Polysubstituted Benzene Concept 4 Video Summary
When naming polysubstituted benzenes, it is essential to denote the locations of substituents using numbers. The carbon atom that carries the principal substituent, which determines the common name, is always assigned the number 1. For instance, in the case of phenol, where the hydroxyl group (–OH) is the primary substituent, it is located at carbon 1. Other substituents, such as bromine and chlorine, are then numbered based on the shortest path from carbon 1. In this example, numbering proceeds clockwise to the bromine at carbon 2 and then to the chlorine at carbon 4. Therefore, the compound is named 2-bromo-4-chlorophenol, reflecting the alphabetical order of the substituents.
Another example is toluene, which consists of a benzene ring attached to a methyl group (–CH3). When additional nitro groups (–NO2) are present, they are also numbered based on their positions relative to the methyl group at carbon 1. In a structure with nitro groups at carbons 2, 3, 4, and 6, the compound is named 2,4,6-trinitrotoluene (TNT). The prefix "tri" indicates the presence of three nitro groups, while "toluene" refers to the benzene ring with the methyl substituent. This compound is notably recognized as the structural formula for dynamite.
In summary, when dealing with polysubstituted benzenes, it is crucial to use numerical designations for the positions of all substituents, ensuring clarity and adherence to IUPAC naming conventions.
Naming Polysubstituted Benzene Example 4
Naming Polysubstituted Benzene Example 4 Video Summary
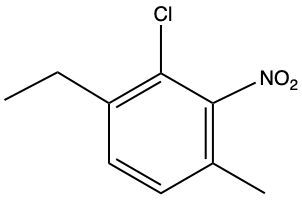
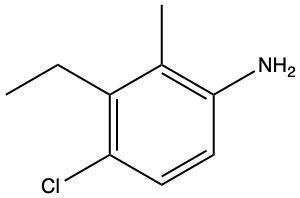
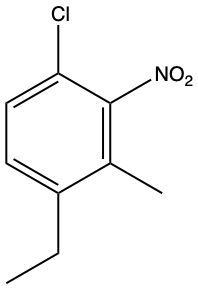
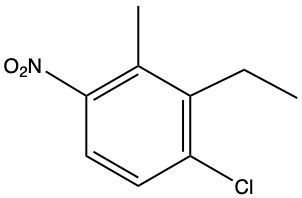
The compound described is a poly-substituted benzene with an amino group (NH2), a chlorine atom (Cl), a fluorine atom (F), and a nitro group (NO2). To name this compound, we start by identifying the primary substituent, which is the amino group, making the base name aniline.
Next, we assign locational numbers to the substituents based on their positions on the benzene ring. The amino group is at position 1. The chlorine is at position 2, the fluorine at position 4, and the nitro group at position 6. When numbering the ring, we prioritize the closest substituents and resolve any ties by considering the alphabetical order of the substituents.
Thus, the full name of the compound, combining the substituents in alphabetical order, is 2-chloro-4-fluoro-6-nitroaniline. This name reflects the positions and types of substituents on the benzene ring without any spaces between the substituents and the base name.
Write an IUPAC name for the following compound.
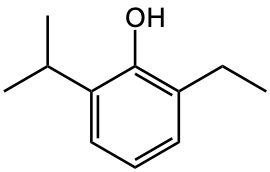
2-ethyl-6-isopropylphenol
2-isopropyl-6-ethylphenol
2-isopropyl-6-ethylanisole
2-ethyl-6-isopropylanisole
Draw a structure for 3-chloro-2-ethyl-6-nitrotoluene.
Do you want more practice?
Here’s what students ask on this topic:
Monosubstituted benzenes are named by identifying the single substituent attached to the benzene ring. Since there is only one substituent, the position on the ring does not need to be specified. The naming convention involves stating the substituent name followed by the word "benzene." For example, if a methyl group (CH3) is attached, the compound is named methylbenzene. This is the simplest form of benzene naming because the ring itself is the parent structure, and only one substituent is present.
In disubstituted benzenes, where two substituents are attached to the benzene ring, the relative positions of these substituents are described using the terms ortho, meta, and para. Ortho (o-) refers to substituents at positions 1 and 2, meta (m-) at positions 1 and 3, and para (p-) at positions 1 and 4 on the benzene ring. These terms provide a simpler way to describe the relative locations instead of using numbers. The mnemonic "order more pizza" helps remember the order: ortho = 1,2; meta = 1,3; para = 1,4. Alternatively, numerical positions can be used for precise naming.
Many monosubstituted benzenes have common names that are widely used alongside their systematic names. For example, when an OH group is attached, the compound is called phenol; an NH2 group gives aniline; a CH3 group results in toluene; an OCH3 group is anisole; an aldehyde group forms benzaldehyde; and a carboxylic acid group forms benzoic acid. These common names are often used because they are simpler and more recognizable. When naming disubstituted benzenes that include one of these groups, the common name becomes the parent name, and the position of the second substituent is indicated using ortho, meta, or para prefixes.
Polysubstituted benzenes, which have three or more substituents, are named by numbering the carbon atoms on the benzene ring to give the substituent that defines the common name the position 1. The other substituents are numbered to minimize the sum of their position numbers. Substituents are then listed alphabetically in the name. For example, in 2,4,6-trinitrotoluene (TNT), the methyl group (CH3) defines the parent name toluene and is assigned carbon 1. The nitro groups (NO2) are located at carbons 2, 4, and 6. This systematic approach ensures clarity and consistency in naming complex benzene derivatives.
The common name in disubstituted and polysubstituted benzenes serves as the parent name when one substituent is a well-known group. For example, in disubstituted benzenes with a methyl group, the parent name is toluene. The other substituents are named and their positions indicated relative to this parent. In polysubstituted benzenes, the substituent that gives the common name is assigned carbon 1, and other substituents are numbered accordingly. This approach simplifies naming by using familiar names like phenol or toluene as the base, making it easier to communicate complex structures.