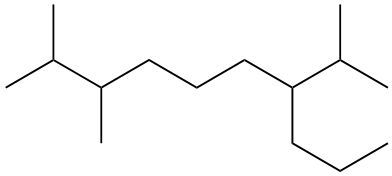
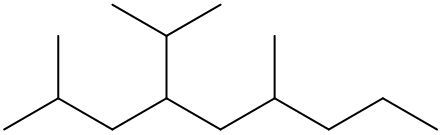
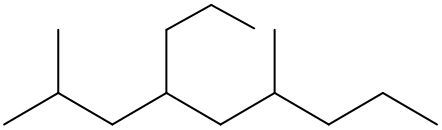
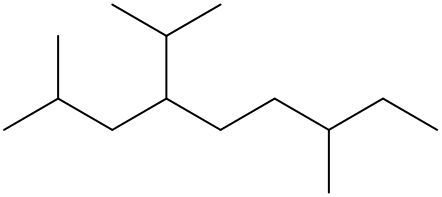
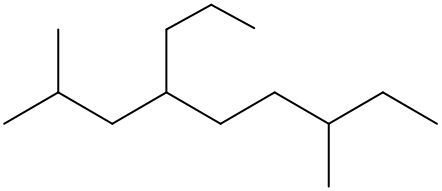
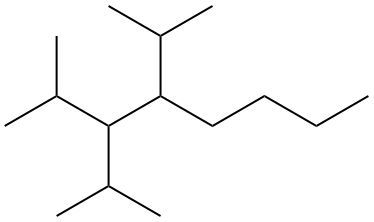
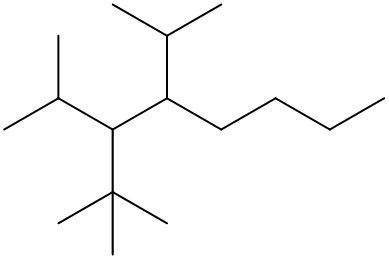
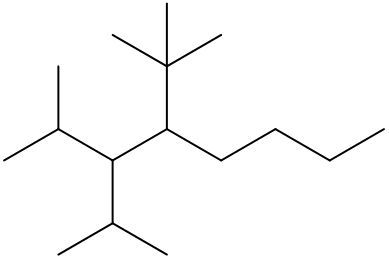
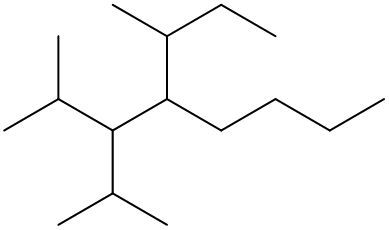
To systematically name an alkane, follow a structured approach that ensures accuracy and clarity. Begin by identifying the longest carbon chain, known as the parent chain. This chain determines the base name of the alkane, which is derived from the number of carbon atoms it contains. For instance, a chain with seven carbon atoms is referred to as "heptane." In cases where there are multiple chains of equal length, select the chain with the most substituents, which are groups attached to the main carbon chain.
When determining the longest carbon chain, consider all possible orientations: left to right, right to left, top to bottom, and bottom to top. This comprehensive examination ensures that you accurately identify the longest chain. Once the longest chain is established, the next step is to name the substituents. Each substituent is assigned a name based on the number of carbon atoms it contains; for example, a one-carbon substituent is a "methyl group," while a two-carbon substituent is an "ethyl group."
Number the carbon atoms in the chain starting from the end closest to the first substituent. If there is a tie, continue numbering based on the proximity of the next substituent. In the event of a further tie, use alphabetical order to determine the numbering sequence. For example, if both an ethyl and a methyl group are present, and they are equidistant from the ends, the ethyl group (E) is prioritized over the methyl group (M) because E comes before M in the alphabet.
Once the substituents are identified and numbered, assign their locations on the parent chain. If there are multiple identical substituents, use numerical prefixes: "di" for two, "tri" for three, and "tetra" for four. For instance, if an ethyl group is located on carbon 3 and a methyl group on carbon 5, the full name of the compound would be structured as follows: "3-ethyl-5-methyl-heptane." Remember to use commas to separate numbers and dashes to separate letters from numbers, while letters remain unseparated.
With practice, the process of naming alkanes and other organic compounds becomes intuitive. Mastery of these naming conventions is essential for effective communication in organic chemistry.