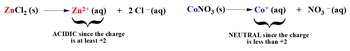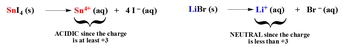Salts are formed when an acid and a base neutralize each other, typically producing water and a second product known as a salt. For example, in the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH), the hydrogen ion (H+) from the acid combines with the hydroxide ion (OH-) from the base to form water (H2O). The sodium ion (Na+) and the chloride ion (Cl-) then combine to form sodium chloride (NaCl), which is the salt produced in this reaction. Salts are ionic compounds consisting of a cation (positive ion) and an anion (negative ion), and their properties can render a solution acidic, basic, or neutral depending on the nature of these ions.
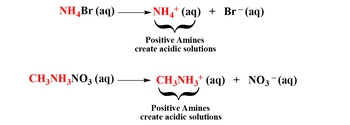
Cations can be categorized into three main groups: transition metals, main group metals, and positive amines. Transition metals are found in the d-block of the periodic table, while main group metals are located in groups 1A, 2A, 3A, and 4A. Positive amines are compounds that contain nitrogen bonded to hydrogen or carbon. The acidity or neutrality of a salt formed from transition metals depends on the charge of the cation. If a transition metal has a charge of +2 or higher, it is considered acidic; if it has a charge less than +2, it is neutral.
For instance, zinc chloride (ZnCl2) contains zinc, which has a +2 charge, making it acidic. Conversely, silver bromide (AgBr) contains silver with a +1 charge, which does not meet the +2 requirement, thus rendering it neutral. Understanding these rules helps predict the behavior of salts in solution, allowing for a deeper comprehension of acid-base chemistry.