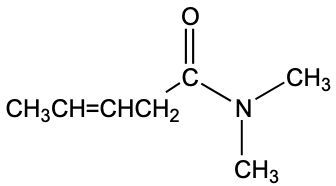
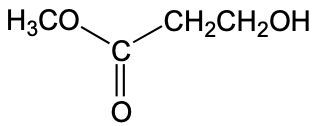
The functional group of a molecule is crucial as it determines the compound's reactivity and characteristics. Understanding how to identify different functional groups is essential in organic chemistry, starting with hydrocarbons, which are compounds composed solely of carbon and hydrogen atoms.
Hydrocarbons can be categorized based on the types of bonds between carbon atoms. The simplest form is the alkane, characterized by carbon atoms connected by single bonds. The general formula for alkanes is CnH2n+2, where n represents the number of carbon atoms. Alkanes can form long chains as long as the carbon atoms remain single bonded to each other and to hydrogen atoms.
Next, we have alkenes, which feature at least one double bond between carbon atoms. The general formula for alkenes is CnH2n. This double bond introduces different reactivity compared to alkanes, making alkenes important in various chemical reactions.
Moving on, alkynes are hydrocarbons that contain at least one triple bond between carbon atoms. The general formula for alkynes is CnH2n-2. The presence of a triple bond significantly alters the properties and reactivity of these compounds.
Lastly, we encounter the benzene ring, also known as an aromatic ring. This structure consists of six carbon atoms arranged in a ring, with alternating double bonds, which can be represented as C6H6. The benzene ring is a fundamental structure in organic chemistry, known for its stability and unique reactivity due to resonance.
In summary, recognizing these functional groups—alkanes, alkenes, alkynes, and benzene rings—is foundational for further studies in organic chemistry, as they dictate the behavior and interactions of various organic compounds.