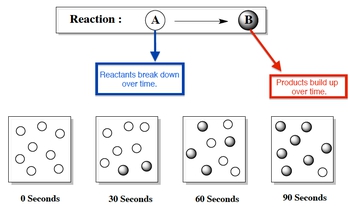
Chemical kinetics is the branch of chemistry that focuses on the rates of chemical reactions, essentially examining how quickly reactants are transformed into products. The term "kinetics" is derived from the Greek word kinesis, which means motion, highlighting the connection between motion and speed. In the context of chemical reactions, the rate refers to the speed at which reactants are consumed and products are formed over a specific period.
Understanding reaction rates is crucial, especially after learning about stoichiometry, which involves calculating limiting reactants and theoretical yields. Now, the challenge is to determine the rates of these reactions, allowing us to quantify how fast the compounds in a balanced chemical equation are reacting. This knowledge is essential for predicting the behavior of chemical systems and optimizing reaction conditions in various applications.