Multiple Choice
Write a name for the following amine.
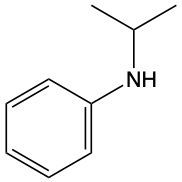
N-isopropyl-N-benzene amine
N-isopropylbenzene amine
N-propylaniline
N-isopropylbenzenamine
 Verified step by step guidance
Verified step by step guidance
 :28m
:28mMaster Common Naming: Amines Concept 1 with a bite sized video explanation from Jules
Start learning