Multiple Choice
Determine if the following reaction represents an oxidation, reduction or neither.
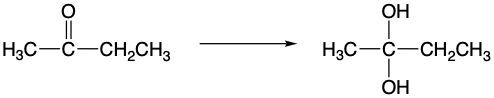
oxidation
reduction
neither
 Verified step by step guidance
Verified step by step guidance
 3:12m
3:12mMaster Identifying Redox Reactions Concept 1 with a bite sized video explanation from Jules
Start learning
