Which of the following will give a positive Tollens' test? (12.4)
a. 1-propanol
b. 2-propanol
c. hexanal
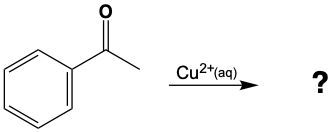
No Reaction
 Verified step by step guidance
Verified step by step guidance
 1:57m
1:57mMaster Oxidation Reactions Concept 1 with a bite sized video explanation from Jules
Start learning
