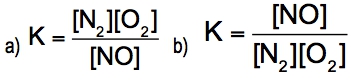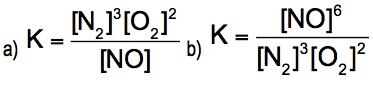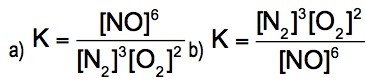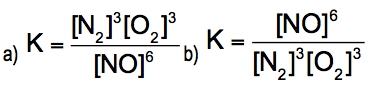Open Question
Oxygen can be converted into ozone by the action of lightning or electric sparks: 3 O2(g) ⇌ 2 O3(g)For this reaction, ∆H = +69kcal/mol (+285 kj/mol) and K = 2.68 x 10^-29 at 25 °C.Are the reactants or the products favored at equilibrium?