Open Question
Consider the following unbalanced equation: (7.4, 7.5, 7.7, 7.8)Al(s) + O₂(g) → Al₂O₃(s)b. Identify the type of reaction.
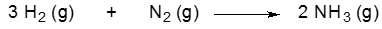
 Verified step by step guidance
Verified step by step guidance
 4:07m
4:07mMaster Types of Chemical Reactions Concept 1 with a bite sized video explanation from Jules
Start learning
