Determine whether the following compounds are acetals or ketals. Draw the structure of the aldehyde or ketone it came from.
c.<IMAGE>
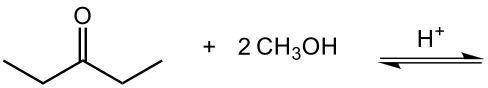
 Verified step by step guidance
Verified step by step guidance
 1:49m
1:49mMaster Hemiacetals and Acetals Concept 1 with a bite sized video explanation from Jules
Start learning
