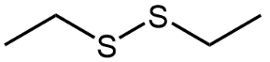
What disulfides would you obtain from oxidation of the following thiols?
a. CH₃CH₂CH₂SH
b. 3-Methyl-1-butanethiol (skunk scent)
 Verified step by step guidance
Verified step by step guidance
 1:49m
1:49mMaster Thiol Redox Reaction Concept 1 with a bite sized video explanation from Jules
Start learning
