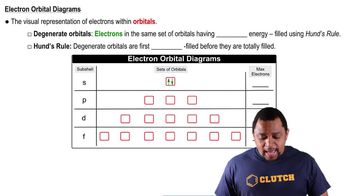
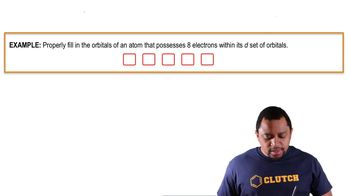
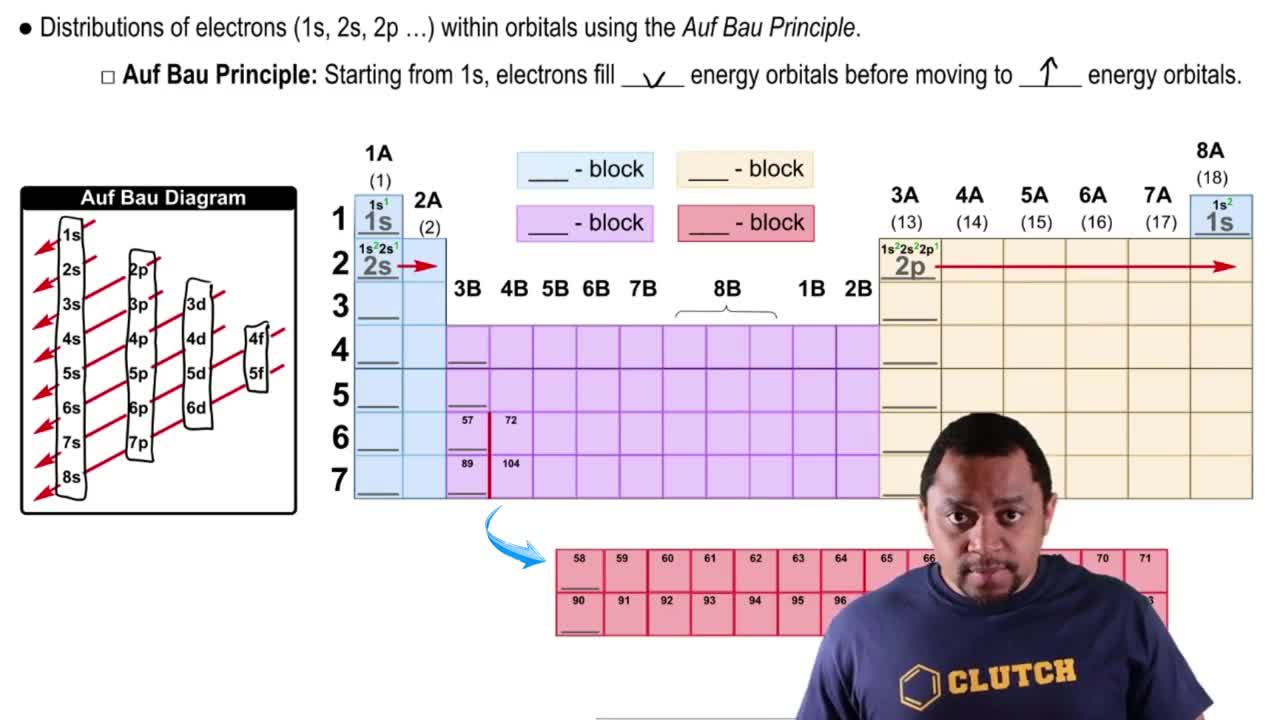
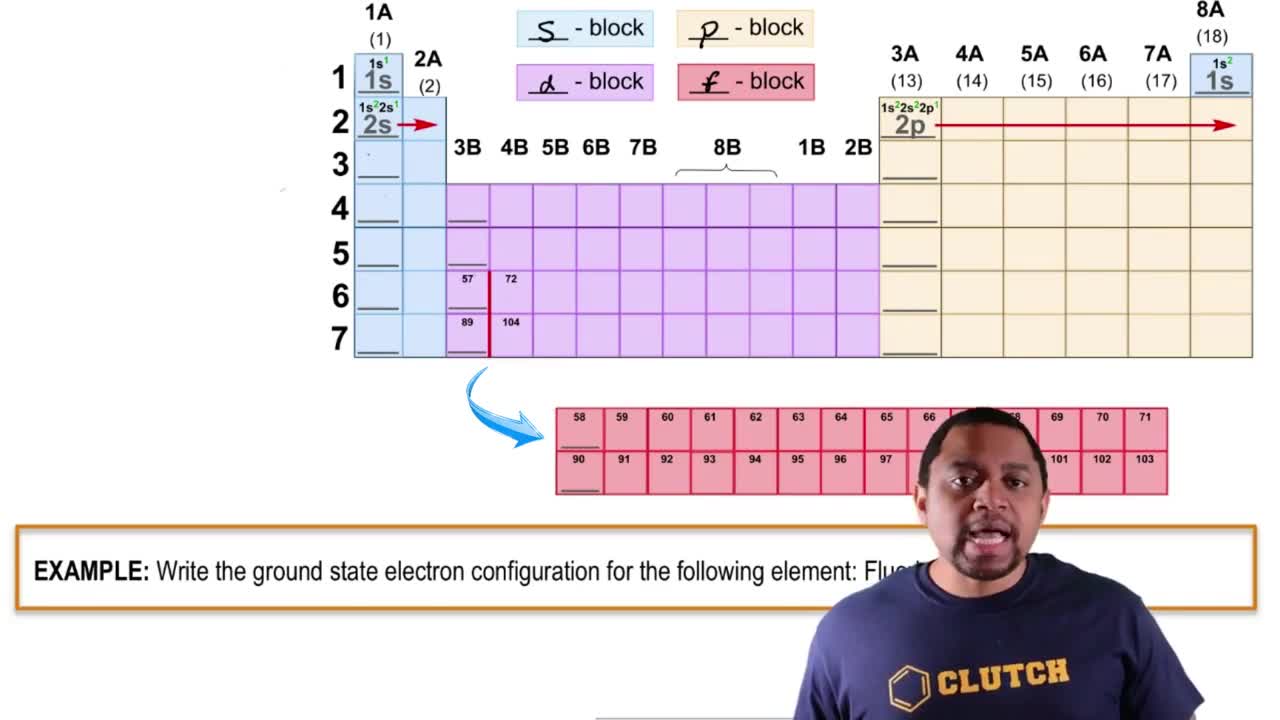
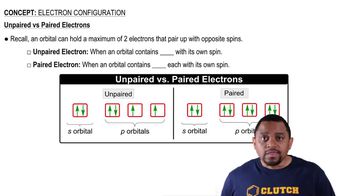
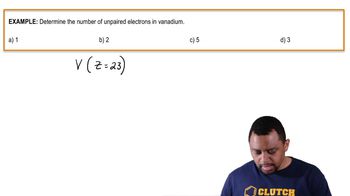
9. Electrons in Atoms and the Periodic Table
The Electron Configuration (Simplified)
9. Electrons in Atoms and the Periodic Table
The Electron Configuration (Simplified)
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
Which electron configuration represents a violation of Hund's Rule?
- Multiple Choice
Which electron configuration represents a violation of the Auf Bau Principle?
- Multiple Choice
Identify the element with the given electron orbital diagram.
- Multiple Choice
Write the electron configuration and electron orbital diagram for the following element:
Sulfur (Z = 16)
- Open QuestionUse the following orbital-filling diagram to show the electron configuration for As:
- Open QuestionUse arrows to show electron pairing in the valence p subshell of a. Sulfurb. Brominec. Silicon
- Open QuestionWhat is wrong with the following electron configurations?a. Ni 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10b. N 1s^2 2p^5c. Si 1s^2 2s^2 2p d. Mg 1s^2 2s^2 2p^6 3s