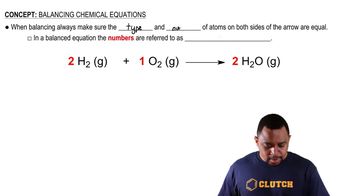
7. Chemical Reactions
Balancing Chemical Equations (Simplified)
7. Chemical Reactions
Balancing Chemical Equations (Simplified)
Practice this topic
- Multiple Choice
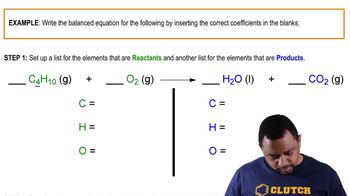
Write the balanced equation for the following by inserting the correct coefficients in the blanks.
- Open Question
Determine the total sum of the coefficients after balancing the following equation.
- Open QuestionWhat is meant by the term 'balanced equation'?
- Open QuestionWhy is it not possible to balance an equation by changing the subscript on a substance, say from H₂O to H₂O₂?
- Open QuestionWhich of the following equations are balanced? Balance those that need it.a. CaC₂+2 H₂O→Ca(OH)₂+C₂H₂ b. C₂H₈N₂+2 N₂O₄→2 N₂+2 CO₂+4 H₂Oc. 3 MgO +2 Fe→Fe₂O₃+3 Mgd. N₂O→N₂+O₂