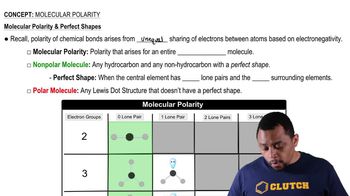
10. Chemical Bonding
Molecular Polarity (Simplified)
10. Chemical Bonding
Molecular Polarity (Simplified)
Practice this topic
- Multiple Choice
Determine if the compound of BCl2F is polar or nonpolar.
- Multiple Choice
Determine if phosphorus trihydride, PH3, is polar or nonpolar.
- Multiple Choice
Determine if difluorine selenide, F2Se, is polar or nonpolar.
- Multiple Choice
Determine if carbon dioxide, CO2, is polar or nonpolar.
- Open QuestionMatch each of the Lewis structures (a to c) with the correct diagram (1 to 3) of its shape, and name the shape; indicate if each molecule is polar or nonpolar. Assume X and Y are nonmetals and all bonds are polar covalent. (6.6, 6.8, 6.9)1. 2. 3. c.
- Open QuestionMatch each of the formulas (a to c) with the correct diagram (1 to 3) of its shape, and name the shape; indicate if each molecule is polar or nonpolar. (6.6, 6.8, 6.9)1. 2. 3. a. PBr₃
- Open QuestionPredict the shape and polarity of each of the following molecules, which have polar covalent bonds: (6.8, 6.9)a. A central atom with three identical bonded atoms and one lone pair.