14. Acids and Bases
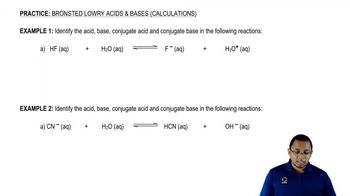
Bronsted Lowry Acid and Base
14. Acids and Bases
Bronsted Lowry Acid and Base
Practice this topic
- Multiple Choice
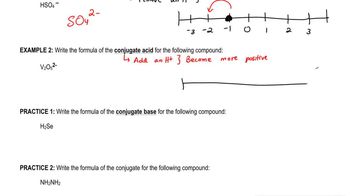
Write the formula of the conjugate base for the following compound:
H2Se
- Multiple Choice
Write the formula of the conjugate for the following compound:
NH2NH2
- Multiple Choice
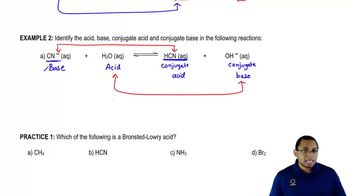
Which of the following is a Bronsted-Lowry acid?
- Multiple Choice
Determine the chemical equation that would result when carbonate, CO32-, reacts with water.
- Open QuestionWhat happens when a strong acid such as HBr is dissolved in water?
- Open QuestionWhat happens when a weak acid such as CH₃CO₂H is dissolved in water?
- Open QuestionWhat happens when a weak base such as NH₃ is dissolved in water?